Profiling Heparan Sulfate-Heavy Metal Ions Interaction Using Electrochemical Techniques
- PMID: 35904207
- PMCID: PMC9804848
- DOI: 10.1002/chem.202202193
Profiling Heparan Sulfate-Heavy Metal Ions Interaction Using Electrochemical Techniques
Abstract
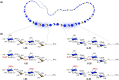
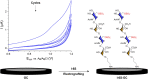
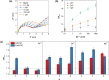
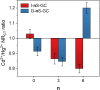
Heparan sulfate glycosaminoglycans provides extracellular matrix defense against heavy metals cytotoxicity. Identifying the precise glycan sequences that bind a particular heavy metal ion is a key for understanding those interactions. Here, electrochemical and surface characterization techniques were used to elucidate the relation between the glycans structural motifs, uronic acid stereochemistry, and sulfation regiochemistry to heavy metal ions binding. A divergent strategy was employed to access a small library of structurally well-defined tetrasaccharides analogs with different sulfation patterns and uronic acid compositions. These tetrasaccharides were electrochemically grafted onto glassy carbon electrodes and their response to heavy metal ions was monitored by electrochemical impedance spectroscopy. Key differences in the binding of Hg(II), Cd(II), and Pb(II) were associated with a combination of the uronic acid type and the sulfation pattern.
Keywords: carbohydrates; electrochemistry; heavy metal ions; sulfation pattern; surface chemistry • uronic acid.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms.Biosens Bioelectron. 2017 Aug 15;94:443-455. doi: 10.1016/j.bios.2017.03.031. Epub 2017 Mar 18. Biosens Bioelectron. 2017. PMID: 28340464 Review.
-
Chitosan-assisted self-assembly of flower-shaped ε-Fe2O3 nanoparticles on screen-printed carbon electrode for Impedimetric detection of Cd2+, Pb2+, and Hg2+ heavy metal ions in various water samples.Int J Biol Macromol. 2024 Apr;265(Pt 2):130867. doi: 10.1016/j.ijbiomac.2024.130867. Epub 2024 Mar 19. Int J Biol Macromol. 2024. PMID: 38508557
-
Exploiting differential electrochemical stripping behaviors of Fe3O4 nanocrystals toward heavy metal ions by crystal cutting.ACS Appl Mater Interfaces. 2014 Aug 13;6(15):12203-13. doi: 10.1021/am501617a. Epub 2014 Jul 18. ACS Appl Mater Interfaces. 2014. PMID: 25014119
-
Electrochemical detection of heavy metal ions in water.Chem Commun (Camb). 2021 Jul 28;57(59):7215-7231. doi: 10.1039/d1cc00983d. Epub 2021 Jul 5. Chem Commun (Camb). 2021. PMID: 34223844 Review.
-
Fullerene-based anodic stripping voltammetry for simultaneous determination of Hg(II), Cu(II), Pb(II) and Cd(II) in foodstuff.Mikrochim Acta. 2018 May 1;185(5):274. doi: 10.1007/s00604-018-2803-9. Mikrochim Acta. 2018. PMID: 29717357
Cited by
-
Surface-Controlled Sialoside-Based Biosensing of Viral and Bacterial Neuraminidases.Langmuir. 2024 Apr 9;40(14):7471-7478. doi: 10.1021/acs.langmuir.3c03943. Epub 2024 Mar 30. Langmuir. 2024. PMID: 38554266 Free PMC article.
-
Impedimetric Characterization of NanA Structural Domains Activity on Sialoside-Containing Interfaces.Langmuir. 2024 Oct 22;40(42):22152-22158. doi: 10.1021/acs.langmuir.4c02620. Epub 2024 Oct 7. Langmuir. 2024. PMID: 39376038 Free PMC article.
-
Biocatalysis versus Molecular Recognition in Sialoside-Selective Neuraminidase Biosensing.ACS Chem Biol. 2023 Mar 17;18(3):605-614. doi: 10.1021/acschembio.2c00913. Epub 2023 Feb 15. ACS Chem Biol. 2023. PMID: 36792550 Free PMC article.
-
Metal-Mediated Il-8 Binding to Heparan Sulfate Evaluated by Electrochemical Impedance Spectroscopy.Chemistry. 2025 Jul 8;31(38):e202501011. doi: 10.1002/chem.202501011. Epub 2025 Jun 18. Chemistry. 2025. PMID: 40485192 Free PMC article.
-
Harnessing glycofluoroforms for impedimetric biosensing.Chem Sci. 2024 Sep 13;15(39):16086-95. doi: 10.1039/d4sc04409f. Online ahead of print. Chem Sci. 2024. PMID: 39282644 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

