Response of multiple hormones to glucose and arginine challenge in T2DM after gastric bypass
- PMID: 35904227
- PMCID: PMC9346340
- DOI: 10.1530/EC-22-0172
Response of multiple hormones to glucose and arginine challenge in T2DM after gastric bypass
Abstract
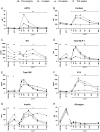
Purpose: In patients with type 2 diabetes mellitus (T2DM), Roux-en-Y gastric bypass (RYGB) leads to beneficial metabolic adaptations, including enhanced incretin secretion, beta-cell function, and systemic insulin sensitivity. We explored the impact of RYGB on pituitary, pancreatic, gut hormones, and cortisol responses to parenteral and enteral nutrient stimulation in patients with obesity and T2DM with repeated sampling up to 2 years after intervention.
Methods: We performed exploratory post hoc analyses in a previously reported randomized trial. Levels of adrenocorticotropic hormone (ACTH), cortisol, growth hormone (GH), glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic peptide (GIP), peptide YY (PYY), ACTH, insulin, and glucagon were measured in 13 patients with T2DM and obesity at four different visits: before and 4, 24, and 104 weeks after RYGB; and in three sequential conditions on the same day: fasting, intravenous arginine challenge, and OGTT.
Results: RYGB surprisingly induced a rise in ACTH, cortisol, and GH levels upon an oral glucose load, together with enhanced GLP-1 and PYY responses. Fasting and post-arginine GH levels were higher after RYGB, whereas insulin, glucagon, GLP-1, GIP, and cortisol were lower. These endocrine adaptations were seen as early as 4 weeks after surgery and were maintained for up to 2 years.
Conclusion: These findings indicate adaptations of glucose sensing mechanisms and responses in multiple endocrine organs after RYGB, involving the gut, pancreatic islets, the pituitary gland, the adrenals, and the brain.
Keywords: HPA-axis; RYGB; diabetes; gut hormones; nutrient challenge.
Figures

Similar articles
-
Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass.Dan Med J. 2015 Apr;62(4):B5057. Dan Med J. 2015. PMID: 25872541 Review.
-
Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones.J Clin Endocrinol Metab. 2013 Nov;98(11):4391-9. doi: 10.1210/jc.2013-2538. Epub 2013 Sep 20. J Clin Endocrinol Metab. 2013. PMID: 24057293
-
Gastric bypass in the pig increases GIP levels and decreases active GLP-1 levels.Peptides. 2017 Apr;90:78-82. doi: 10.1016/j.peptides.2017.02.009. Epub 2017 Feb 24. Peptides. 2017. PMID: 28242256
-
Responses of gut and pancreatic hormones, bile acids, and fibroblast growth factor-21 differ to glucose, protein, and fat ingestion after gastric bypass surgery.Am J Physiol Gastrointest Liver Physiol. 2020 Apr 1;318(4):G661-G672. doi: 10.1152/ajpgi.00265.2019. Epub 2020 Feb 18. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 32068442
-
EndoBarrier gastrointestinal liner. Delineation of underlying mechanisms and clinical effects.Dan Med J. 2016 Nov;63(11):B5309. Dan Med J. 2016. PMID: 27808040 Review.
Cited by
-
Short-term effects of obesity surgery versus low-energy diet on body composition and tissue-specific glucose uptake: a randomised clinical study using whole-body integrated 18F-FDG-PET/MRI.Diabetologia. 2024 Jul;67(7):1399-1412. doi: 10.1007/s00125-024-06150-3. Epub 2024 Apr 24. Diabetologia. 2024. PMID: 38656372 Free PMC article. Clinical Trial.
-
Hormonal adaptations to weight loss: Responses to an oral glucose load 4 weeks after obesity surgery and low-energy diet.Diabetes Obes Metab. 2025 Sep;27(9):4836-4846. doi: 10.1111/dom.16526. Epub 2025 Jun 16. Diabetes Obes Metab. 2025. PMID: 40521749 Free PMC article. Clinical Trial.
-
Similar early metabolic changes induced by dietary weight loss or bariatric surgery. Reply to Taylor R [letter].Diabetologia. 2024 Nov;67(11):2605-2607. doi: 10.1007/s00125-024-06276-4. Epub 2024 Sep 5. Diabetologia. 2024. PMID: 39235459 No abstract available.
References
-
- Purnell JQ, Dewey EN, Laferrère B, Selzer F, Flum DR, Mitchell JE, Pomp A, Pories WJ, Inge T, Courcoulas A.et al. Diabetes remission status during seven-year follow-up of the longitudinal assessment of bariatric surgery study. Journal of Clinical Endocrinology and Metabolism 2021106774–788. (10.1210/clinem/dgaa849) - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

