Functional consequences of allotypic polymorphisms in human immunoglobulin G subclasses
- PMID: 35904629
- PMCID: PMC9845132
- DOI: 10.1007/s00251-022-01272-7
Functional consequences of allotypic polymorphisms in human immunoglobulin G subclasses
Abstract
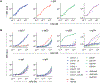
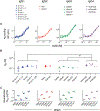
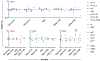
Heritable polymorphisms within the human IgG locus, collectively termed allotypes, have often been linked by statistical associations, but rarely mechanistically, to a wide range of disease states. One potential explanation for these associations is that IgG allotype alters host cell receptors' affinity for IgG, dampening or enhancing an immune response depending on the nature of the change and the receptors. In this work, a panel of allotypic antibody variants were evaluated using multiplexed, label-free biophysical methods and cell-based functional assays to determine what effect, if any, human IgG polymorphisms have on antibody function. While we observed several differences in FcγR affinity among allotypes, there was little evidence of dramatically altered FcγR-based effector function or antigen recognition activity associated with this aspect of genetic variability.
Keywords: Allotype; Effector function; Fc receptor; IgG; Immunuglobulin; Polymorphism.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Competing Interests
The authors declare to Competing Financial or Non-Financial Interests.
Figures



Similar articles
-
Impact of structural modifications of IgG antibodies on effector functions.Front Immunol. 2024 Jan 8;14:1304365. doi: 10.3389/fimmu.2023.1304365. eCollection 2023. Front Immunol. 2024. PMID: 38259472 Free PMC article. Review.
-
FcγR Binding and ADCC Activity of Human IgG Allotypes.Front Immunol. 2020 May 6;11:740. doi: 10.3389/fimmu.2020.00740. eCollection 2020. Front Immunol. 2020. PMID: 32435243 Free PMC article.
-
Susceptibility to Plasmodium falciparum Malaria: Influence of Combined Polymorphisms of IgG3 Gm Allotypes and Fc Gamma Receptors IIA, IIIA, and IIIB.Front Immunol. 2020 Dec 23;11:608016. doi: 10.3389/fimmu.2020.608016. eCollection 2020. Front Immunol. 2020. PMID: 33424858 Free PMC article.
-
Role of immune recognition in latent allotype induction and clearance. Evidence for an allotypic network.J Exp Med. 1981 Jan 1;153(1):196-206. doi: 10.1084/jem.153.1.196. J Exp Med. 1981. PMID: 7452153 Free PMC article.
-
IgG-effector functions: "the good, the bad and the ugly".Immunol Lett. 2014 Aug;160(2):139-44. doi: 10.1016/j.imlet.2014.01.015. Epub 2014 Feb 1. Immunol Lett. 2014. PMID: 24495619 Review.
Cited by
-
Identification of IgG1 and IgG3 Allotypes by PCR and Sanger Sequencing.Methods Mol Biol. 2024;2826:201-218. doi: 10.1007/978-1-0716-3950-4_15. Methods Mol Biol. 2024. PMID: 39017895
-
Impact of structural modifications of IgG antibodies on effector functions.Front Immunol. 2024 Jan 8;14:1304365. doi: 10.3389/fimmu.2023.1304365. eCollection 2023. Front Immunol. 2024. PMID: 38259472 Free PMC article. Review.
-
Squamate reptiles may have compensated for the lack of γδTCR with a duplication of the TRB locus.Front Immunol. 2025 Jan 9;15:1524471. doi: 10.3389/fimmu.2024.1524471. eCollection 2024. Front Immunol. 2025. PMID: 39850903 Free PMC article.
-
Multivariate analysis of FcR-mediated NK cell functions identifies unique clustering among humans and rhesus macaques.Front Immunol. 2023 Dec 6;14:1260377. doi: 10.3389/fimmu.2023.1260377. eCollection 2023. Front Immunol. 2023. PMID: 38124734 Free PMC article.
-
Afucosylation of HLA-specific IgG1 as a potential predictor of antibody pathogenicity in kidney transplantation.Cell Rep Med. 2022 Nov 15;3(11):100818. doi: 10.1016/j.xcrm.2022.100818. Cell Rep Med. 2022. PMID: 36384101 Free PMC article.
References
-
- van der Zee JS, van Swieten P, and Aalberse RC, Serologic aspects of IgG4 antibodies. II. IgG4 antibodies form small, nonprecipitating immune complexes due to functional monovalency. J Immunol 137 (1986) 3566–71. - PubMed
-
- Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, et al., Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 113 (2009) 3716–25. - PubMed
-
- Grubb R, Agglutination of erythrocytes coated with incomplete anti-Rh by certain rheumatoid arthritic sera and some other sera; the existence of human serum groups. Acta Pathol Microbiol Scand 39 (1956) 195–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

