Update on Melasma-Part I: Pathogenesis
- PMID: 35904706
- PMCID: PMC9464278
- DOI: 10.1007/s13555-022-00779-x
Update on Melasma-Part I: Pathogenesis
Abstract
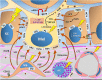
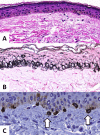
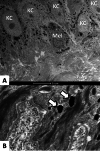
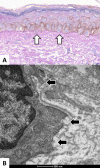
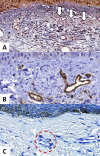
Melasma is a multifactorial dyschromia that results from exposure to external factors (such as solar radiation) and hormonal factors (such as sex hormones and pregnancy), as well as skin inflammation (such as contact dermatitis and esthetic procedures), in genetically predisposed individuals. Beyond hyperfunctional melanocytes, skin with melasma exhibits a series of structural and functional alterations in the epidermis, basement membrane, and upper dermis that interact to elicit and sustain a focal hypermelanogenic phenotype. Evolution in the knowledge of the genetic basis of melasma and the cutaneous response to solar radiation, as well as the roles of endocrine factors, antioxidant system, endothelium proliferation, fibroblast senescence, mast cell degranulation, autophagy deficits of the melanocyte, and the paracrine regulation of melanogenesis, will lead to the development of new treatments and preventive strategies. This review presents current knowledge on these aspects of the pathogenesis of melasma and discusses the effects of specific treatments and future research on these issues.
Keywords: Melanin; Melanocytes; Melasma; Pathogenesis; Photoaging; UV radiation.
© 2022. The Author(s).
Figures

References
-
- Esposito ACC, Brianezi G, de Souza NP, Miot LDB, Miot HA. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32(2):101–108. doi: 10.5021/ad.2020.32.2.101. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

