Safety and Immunogenicity of a Respiratory Syncytial Virus Prefusion F (RSVPreF3) Candidate Vaccine in Older Adults: Phase 1/2 Randomized Clinical Trial
- PMID: 35904987
- PMCID: PMC10044090
- DOI: 10.1093/infdis/jiac327
Safety and Immunogenicity of a Respiratory Syncytial Virus Prefusion F (RSVPreF3) Candidate Vaccine in Older Adults: Phase 1/2 Randomized Clinical Trial
Abstract
Background: The aim of this study was to investigate safety and immunogenicity of vaccine formulations against respiratory syncytial virus (RSV) containing the stabilized prefusion conformation of RSV fusion protein (RSVPreF3).
Methods: This phase 1/2, randomized controlled, observer-blind study enrolled 48 young adults (YAs; aged 18-40 years) and 1005 older adults (OAs; aged 60-80 years) between January and August 2019. Participants were randomized into equally sized groups to receive 2 doses of unadjuvanted (YAs and OAs) or AS01-adjuvanted (OAs) vaccine or placebo 2 months apart. Vaccine safety and immunogenicity were assessed until 1 month (YAs) or 12 months (OAs) after second vaccination.
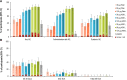
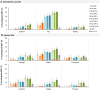
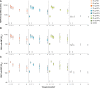
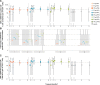
Results: The RSVPreF3 vaccines boosted humoral (RSVPreF3-specific immunoglobulin G [IgG] and RSV-A neutralizing antibody) responses, which increased in an antigen concentration-dependent manner and were highest after dose 1. Compared to prevaccination, the geometric mean frequencies of polyfunctional CD4+ T cells increased after each dose and were significantly higher in adjuvanted than unadjuvanted vaccinees. Postvaccination immune responses persisted until end of follow-up. Solicited adverse events were mostly mild to moderate and transient. Despite a higher observed reactogenicity of AS01-containing vaccines, no safety concerns were identified for any assessed formulation.
Conclusions: Based on safety and immunogenicity profiles, the AS01E-adjuvanted vaccine containing 120 μg of RSVPreF3 was selected for further clinical development.
Clinical trials registration: NCT03814590.
Keywords: AS01 adjuvant; F protein; RSV neutralizing antibodies; cell-mediated immunity; respiratory syncytial virus.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. D. C., M.-P. D., M. d. H., N. D. S., N. D., L. F., V. H., J. K., N. M., B. S., F. S., J. T., C. V. A., and C. Ve. are/were employees of the GSK group of companies at the time of the study conduct. C. Va. is currently an employee of the GSK group of companies. D. C., M.-P. D., M. d. H., N. D. S., N. D., B. S., F. S., and C. Ve. hold shares from the GSK group of companies as part of their past/current employee remuneration. F. S. is currently an employee of Janssen Pharmaceutical Companies of Johnson & Johnson and holds restricted shares from Johnson & Johnson as part of his employee remuneration. All current/previous employees of the GSK groups of companies declare financial and nonfinancial relationships and activities. C. P. A., E. K., I. L.-R., K. S., and C. Va. report grant/research support from the GSK group of companies to their institution for study conduct and, except for C. Va., they have no nonfinancial relationships and activities to declare. E. K. has served as consultant, in advisory boards, in speaker’s bureaus, or received travel reimbursement from Amphastar, AstraZeneca, Boehringer Ingelheim, Forest, Cipla, Chiesi, GSK, Mylan, Novartis, Sunovion, Teva, Pearl Pharmaceuticals, and Theravance. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
Similar articles
-
Safety and Immunogenicity of a Revaccination With a Respiratory Syncytial Virus Prefusion F Vaccine in Older Adults: A Phase 2b Study.J Infect Dis. 2024 Feb 14;229(2):355-366. doi: 10.1093/infdis/jiad321. J Infect Dis. 2024. PMID: 37699064 Free PMC article.
-
Immunogenicity and Safety Following 1 Dose of AS01E-Adjuvanted Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults: A Phase 3 Trial.J Infect Dis. 2024 Jul 25;230(1):e102-e110. doi: 10.1093/infdis/jiad546. J Infect Dis. 2024. PMID: 39052726 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of a respiratory syncytial virus prefusion F protein (RSVPreF3) candidate vaccine in older Japanese adults: A phase I, randomized, observer-blind clinical trial.Respir Investig. 2023 Mar;61(2):261-269. doi: 10.1016/j.resinv.2022.11.003. Epub 2023 Jan 12. Respir Investig. 2023. PMID: 36641341 Clinical Trial.
-
Vaccines for Respiratory Syncytial Virus Prevention in Older Adults.Ann Pharmacother. 2024 Dec;58(12):1218-1228. doi: 10.1177/10600280241241049. Epub 2024 Apr 2. Ann Pharmacother. 2024. PMID: 38563554 Review.
-
Efficacy, immunogenicity and safety of respiratory syncytial virus prefusion F vaccine: systematic review and meta-analysis.BMC Public Health. 2024 May 6;24(1):1244. doi: 10.1186/s12889-024-18748-8. BMC Public Health. 2024. PMID: 38711074 Free PMC article.
Cited by
-
Vaccines' New Era-RNA Vaccine.Viruses. 2023 Aug 18;15(8):1760. doi: 10.3390/v15081760. Viruses. 2023. PMID: 37632102 Free PMC article. Review.
-
Structure and antigenicity of divergent Henipavirus fusion glycoproteins.Nat Commun. 2023 Jun 16;14(1):3577. doi: 10.1038/s41467-023-39278-8. Nat Commun. 2023. PMID: 37328468 Free PMC article.
-
Noninferior Immunogenicity and Consistent Safety of Respiratory Syncytial Virus Prefusion F Protein Vaccine in Adults 50-59 Years Compared to ≥60 Years of Age.Clin Infect Dis. 2024 Oct 15;79(4):1074-1084. doi: 10.1093/cid/ciae364. Clin Infect Dis. 2024. PMID: 39099093 Free PMC article. Clinical Trial.
-
Immunogenicity, safety, and tolerability of a β-glucan-CpG-adjuvanted respiratory syncytial virus vaccine in Japanese healthy participants aged 60 to 80 years: A phase 2, randomized, double-blind, dose-finding study.Hum Vaccin Immunother. 2025 Dec;21(1):2489900. doi: 10.1080/21645515.2025.2489900. Epub 2025 Apr 21. Hum Vaccin Immunother. 2025. PMID: 40257186 Free PMC article. Clinical Trial.
-
Developing Correlates of Protection for Vaccines Is Needed More than Ever-Influenza, COVID-19 and RSV Infection.Viruses. 2024 Oct 25;16(11):1671. doi: 10.3390/v16111671. Viruses. 2024. PMID: 39599786 Free PMC article.
References
-
- Simoes EA. Respiratory syncytial virus infection. Lancet 1999; 354:847–52. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

