Antioxidative enzyme NAD(P)H quinone oxidoreductase 1 (NQO1) modulates the differentiation of Th17 cells by regulating ROS levels
- PMID: 35905076
- PMCID: PMC9337673
- DOI: 10.1371/journal.pone.0272090
Antioxidative enzyme NAD(P)H quinone oxidoreductase 1 (NQO1) modulates the differentiation of Th17 cells by regulating ROS levels
Abstract
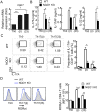
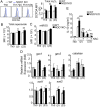
NAD(P)H quinone oxidoreductase 1 (NQO1) is a flavoprotein that catalyzes two-electron reduction of quinone to hydroquinone by using nicotinamide adenine dinucleotide (NADPH), and functions as a scavenger for reactive oxygen species (ROS). The function of NQO1 in the immune response is not well known. In the present study, we demonstrated that Nqo1-deficient T cells exhibited reduced induction of T helper 17 cells (Th17) in vitro during Th17(23)- and Th17(β)- skewing conditions. Nqo1-deficient mice showed ameliorated symptoms in a Th17-dependent autoimmune Experimental autoimmune encephalomyelitis (EAE) model. Impaired Th17-differentiation was caused by overproduction of the immunosuppressive cytokine, IL-10. Increased IL-10 production in Nqo1-deficient Th17 cells was associated with elevated intracellular Reactive oxygen species (ROS) levels. Furthermore, overproduction of IL-10 in Th17 (β) cells was responsible for the ROS-dependent increase of c-avian musculoaponeurotic fibrosarcoma (c-maf) expression, despite the lack of dependency of c-maf in Th17(23) cells. Taken together, the results reveal a novel role of NQO1 in promoting Th17 development through the suppression of ROS mediated IL-10 production.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

