The RNA-binding protein Puf5 and the HMGB protein Ixr1 contribute to cell cycle progression through the regulation of cell cycle-specific expression of CLB1 in Saccharomyces cerevisiae
- PMID: 35905103
- PMCID: PMC9365169
- DOI: 10.1371/journal.pgen.1010340
The RNA-binding protein Puf5 and the HMGB protein Ixr1 contribute to cell cycle progression through the regulation of cell cycle-specific expression of CLB1 in Saccharomyces cerevisiae
Abstract
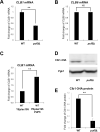
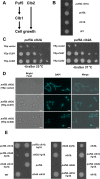
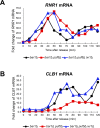
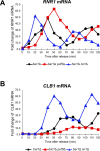
Puf5, a Puf-family RNA-binding protein, binds to 3´ untranslated region of target mRNAs and negatively regulates their expression in Saccharomyces cerevisiae. The puf5Δ mutant shows pleiotropic phenotypes including a weakened cell wall, a temperature-sensitive growth, and a shorter lifespan. To further analyze a role of Puf5 in cell growth, we searched for a multicopy suppressor of the temperature-sensitive growth of the puf5Δ mutant in this study. We found that overexpression of CLB2 encoding B-type cyclin suppressed the temperature-sensitive growth of the puf5Δ mutant. The puf5Δ clb2Δ double mutant displayed a severe growth defect, suggesting that Puf5 positively regulates the expression of a redundant factor with Clb2 in cell cycle progression. We found that expression of CLB1 encoding a redundant B-type cyclin was decreased in the puf5Δ mutant, and that this decrease of the CLB1 expression contributed to the growth defect of the puf5Δ clb2Δ double mutant. Since Puf5 is a negative regulator of the gene expression, we hypothesized that Puf5 negatively regulates the expression of a factor that represses CLB1 expression. We found such a repressor, Ixr1, which is an HMGB (High Mobility Group box B) protein. Deletion of IXR1 restored the decreased expression of CLB1 caused by the puf5Δ mutation and suppressed the growth defect of the puf5Δ clb2Δ double mutant. The expression of IXR1 was negatively regulated by Puf5 in an IXR1 3´ UTR-dependent manner. Our results suggest that IXR1 mRNA is a physiologically important target of Puf5, and that Puf5 and Ixr1 contribute to the cell cycle progression through the regulation of the cell cycle-specific expression of CLB1.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures








Similar articles
-
The RNA-binding protein Puf5 and the HMGB protein Ixr1 regulate cell cycle-specific expression of CLB1 and CLB2 in Saccharomyces cerevisiae.PLoS One. 2025 Feb 3;20(2):e0316433. doi: 10.1371/journal.pone.0316433. eCollection 2025. PLoS One. 2025. PMID: 39899527 Free PMC article.
-
Regulation of LRG1 expression by RNA-binding protein Puf5 in the budding yeast cell wall integrity pathway.Genes Cells. 2018 Dec;23(12):988-997. doi: 10.1111/gtc.12646. Epub 2018 Oct 31. Genes Cells. 2018. PMID: 30281869
-
Ixr1 is required for the expression of the ribonucleotide reductase Rnr1 and maintenance of dNTP pools.PLoS Genet. 2011 May;7(5):e1002061. doi: 10.1371/journal.pgen.1002061. Epub 2011 May 5. PLoS Genet. 2011. PMID: 21573136 Free PMC article.
-
Expression Dynamics and Genetic Compensation of Cell Cycle Paralogues in Saccharomyces cerevisiae.Cells. 2025 Mar 11;14(6):412. doi: 10.3390/cells14060412. Cells. 2025. PMID: 40136661 Free PMC article.
-
The DNA binding high mobility group box protein family functionally binds RNA.Wiley Interdiscip Rev RNA. 2023 Sep-Oct;14(5):e1778. doi: 10.1002/wrna.1778. Epub 2023 Jan 16. Wiley Interdiscip Rev RNA. 2023. PMID: 36646476 Free PMC article. Review.
Cited by
-
The RNA-binding protein Puf5 and the HMGB protein Ixr1 regulate cell cycle-specific expression of CLB1 and CLB2 in Saccharomyces cerevisiae.PLoS One. 2025 Feb 3;20(2):e0316433. doi: 10.1371/journal.pone.0316433. eCollection 2025. PLoS One. 2025. PMID: 39899527 Free PMC article.
-
Engineering transcriptional regulatory networks for improving second-generation fuel ethanol production in Saccharomyces cerevisiae.Synth Syst Biotechnol. 2024 Oct 28;10(1):207-217. doi: 10.1016/j.synbio.2024.10.006. eCollection 2025. Synth Syst Biotechnol. 2024. PMID: 39558946 Free PMC article. Review.
-
Thanksgiving to Yeast, the HMGB Proteins History from Yeast to Cancer.Microorganisms. 2023 Apr 11;11(4):993. doi: 10.3390/microorganisms11040993. Microorganisms. 2023. PMID: 37110415 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

