Viraemic-time predicts mortality among people living with HIV on second-line antiretroviral treatment in Myanmar: A retrospective cohort study
- PMID: 35905123
- PMCID: PMC9337705
- DOI: 10.1371/journal.pone.0271910
Viraemic-time predicts mortality among people living with HIV on second-line antiretroviral treatment in Myanmar: A retrospective cohort study
Erratum in
-
Correction: Viraemic-time predicts mortality among people living with HIV on second-line antiretroviral treatment in Myanmar: A retrospective cohort study.PLoS One. 2024 Feb 22;19(2):e0299460. doi: 10.1371/journal.pone.0299460. eCollection 2024. PLoS One. 2024. PMID: 38386670 Free PMC article.
Abstract
Introduction: Despite HIV viral load (VL) monitoring being serial, most studies use a cross-sectional design to evaluate the virological status of a cohort. The objective of our study was to use a simplified approach to calculate viraemic-time: the proportion of follow-up time with unsuppressed VL above the limit of detection. We estimated risk factors for higher viraemic-time and whether viraemic-time predicted mortality in a second-line antiretroviral treatment (ART) cohort in Myanmar.
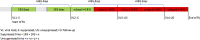
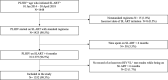
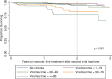
Methods: We conducted a retrospective cohort analysis of people living with HIV (PLHIV) who received second-line ART for a period >6 months and who had at least two HIV VL test results between 01 January 2014 and 30 April 2018. Fractional logistic regression assessed risk factors for having higher viraemic-time and Cox proportional hazards regression assessed the association between viraemic-time and mortality. Kaplan-Meier curves were plotted to illustrate survival probability for different viraemic-time categories.
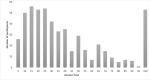
Results: Among 1,352 participants, 815 (60.3%) never experienced viraemia, and 172 (12.7%), 214 (15.8%), and 80 (5.9%) participants were viraemic <20%, 20-49%, and 50-79% of their total follow-up time, respectively. Few (71; 5.3%) participants were ≥80% of their total follow-up time viraemic. The odds for having higher viraemic-time were higher among people with a history of injecting drug use (aOR 2.01, 95% CI 1.30-3.10, p = 0.002), sex workers (aOR 2.10, 95% CI 1.11-4.00, p = 0.02) and patients treated with lopinavir/ritonavir (vs. atazanavir; aOR 1.53, 95% CI 1.12-2.10, p = 0.008). Viraemic-time was strongly associated with mortality hazard among those with 50-79% and ≥80% viraemic-time (aHR 2.92, 95% CI 1.21-7.10, p = 0.02 and aHR 2.71, 95% CI 1.22-6.01, p = 0.01). This association was not observed in those with viraemic-time <50%.
Conclusions: Key populations were at risk for having a higher viraemic-time on second-line ART. Viraemic-time predicts clinical outcomes. Differentiated services should target subgroups at risk for a higher viraemic-time to control both HIV transmission and mortality.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Systematic review on cumulative HIV viraemia among people living with HIV receiving antiretroviral treatment and its association with mortality and morbidity.Int Health. 2024 May 1;16(3):261-278. doi: 10.1093/inthealth/ihad093. Int Health. 2024. PMID: 37823452 Free PMC article.
-
Predictors of virological failure among people living with HIV receiving first line antiretroviral treatment in Myanmar: retrospective cohort analysis.AIDS Res Ther. 2021 Apr 21;18(1):16. doi: 10.1186/s12981-021-00336-0. AIDS Res Ther. 2021. PMID: 33882962 Free PMC article.
-
Low-level HIV viraemia during antiretroviral therapy: Longitudinal patterns and predictors of viral suppression.HIV Med. 2024 Jan;25(1):107-116. doi: 10.1111/hiv.13541. Epub 2023 Sep 18. HIV Med. 2024. PMID: 37721192
-
Factors associated with human immunodeficiency virus-1 low-level viremia and its impact on virological and immunological outcomes: A retrospective cohort study in Beijing, China.HIV Med. 2022 Mar;23 Suppl 1:72-83. doi: 10.1111/hiv.13251. HIV Med. 2022. PMID: 35293102
-
DETECTION OF VIRAEMIC HBV INFECTION – to guide who to treat or not treat.WHO Guidelines on Hepatitis B and C Testing. Geneva: World Health Organization; 2017 Feb. 9. WHO Guidelines on Hepatitis B and C Testing. Geneva: World Health Organization; 2017 Feb. 9. PMID: 30418715 Free Books & Documents. Review. No abstract available.
Cited by
-
Systematic review on cumulative HIV viraemia among people living with HIV receiving antiretroviral treatment and its association with mortality and morbidity.Int Health. 2024 May 1;16(3):261-278. doi: 10.1093/inthealth/ihad093. Int Health. 2024. PMID: 37823452 Free PMC article.
-
Correction: Viraemic-time predicts mortality among people living with HIV on second-line antiretroviral treatment in Myanmar: A retrospective cohort study.PLoS One. 2024 Feb 22;19(2):e0299460. doi: 10.1371/journal.pone.0299460. eCollection 2024. PLoS One. 2024. PMID: 38386670 Free PMC article.
-
Outcomes of severely ill patients with AIDS treated with efavirenz or dolutegravir: a multicenter, observational study.Front Med (Lausanne). 2024 Feb 28;11:1302710. doi: 10.3389/fmed.2024.1302710. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38482527 Free PMC article.
-
Treatment Outcomes After Switching to Second-Line Anti-Retroviral Therapy: Results From the Thai National Treatment Program.J Int Assoc Provid AIDS Care. 2023 Jan-Dec;22:23259582231220513. doi: 10.1177/23259582231220513. J Int Assoc Provid AIDS Care. 2023. PMID: 38115729 Free PMC article.
References
-
- Estill J, Ford N, Salazar-Vizcaya L, Haas AD, Blaser N, Habiyambere V, et al.. The need for second-line antiretroviral therapy in adults in sub-Saharan Africa up to 2030: a mathematical modelling study. Lancet HIV. 2016;3(3):e132–9. Epub 2016/03/05. doi: 10.1016/S2352-3018(16)00016-3 - DOI - PMC - PubMed
-
- World Heatlh Organization. Consolidated Guidelines on The Use Of Antiretroviral Drugs for Treating And Preventing HIV Infection Geneva: World Heatlh Organization; 2013 [cited 2021 May 25]; Available from: https://www.who.int/hiv/pub/guidelines/arv2013/download/en/.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

