Understanding the impact process of vaccine adoption for COVID-19
- PMID: 35905384
- PMCID: PMC9746427
- DOI: 10.1080/21645515.2022.2099166
Understanding the impact process of vaccine adoption for COVID-19
Abstract
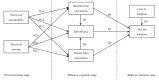
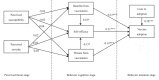
Vaccination for the novel coronavirus disease 2019 (COVID-19) provides an effective approach for the general improvement of social safety and individual health. To date, few studies have analyzed the adoption of COVID-19 vaccines from an entire impact process perspective. Using the health belief model (HBM) and the valence theory, this research evaluates the impact process of vaccine adoption for COVID-19. The respondents in this study were individuals who have been vaccinated in China. The effective sample included 595 individuals. Four valuable and novel findings are identified through this research. First, neither perceived susceptibility nor perceived severity has a statistically significant impact on the benefits from vaccination, threats from vaccination and self-efficacy. Second, benefits from vaccination produce a significant positive effect on self-efficacy and vaccine adoption. Third, threats from vaccination produce a significant negative effect on self-efficacy and vaccine adoption. Fourth, both self-efficacy and cues to adoption produce a significantly positive impact on vaccine adoption. Our theoretical model, which is the main contribution of this research, indicates that individual vaccine adoption is simply a process that leads from behavioral cognition to behavioral intention, rather than from psychological perception to behavioral cognition and then from behavioral cognition to behavioral intention.
Keywords: Novel coronavirus disease 2019 (COVID-19); health belief model (HBM); self-efficacy; vaccine adoption; valence theory.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
Understanding the continuous vaccination of the COVID-19 vaccine: an empirical study from China.Hum Vaccin Immunother. 2021 Dec 2;17(12):4954-4963. doi: 10.1080/21645515.2021.2013080. Epub 2021 Dec 10. Hum Vaccin Immunother. 2021. PMID: 34893013 Free PMC article.
-
The impact of vaccine hesitation on the intentions to get COVID-19 vaccines: The use of the health belief model and the theory of planned behavior model.Front Public Health. 2022 Oct 14;10:882909. doi: 10.3389/fpubh.2022.882909. eCollection 2022. Front Public Health. 2022. PMID: 36311575 Free PMC article.
-
The intention to get a COVID-19 vaccine among the students of health science in Vietnam.Hum Vaccin Immunother. 2021 Dec 2;17(12):4823-4828. doi: 10.1080/21645515.2021.1981726. Epub 2021 Oct 8. Hum Vaccin Immunother. 2021. PMID: 34623931 Free PMC article.
-
Intention to receive COVID-19 vaccine and its health belief model (HBM)-based predictors: A systematic review and meta-analysis.Hum Vaccin Immunother. 2023 Dec 31;19(1):2207442. doi: 10.1080/21645515.2023.2207442. Epub 2023 May 12. Hum Vaccin Immunother. 2023. PMID: 37170620 Free PMC article.
-
How Well the Constructs of Health Belief Model Predict Vaccination Intention: A Systematic Review on COVID-19 Primary Series and Booster Vaccines.Vaccines (Basel). 2023 Apr 7;11(4):816. doi: 10.3390/vaccines11040816. Vaccines (Basel). 2023. PMID: 37112728 Free PMC article. Review.
Cited by
-
Predicting COVID-19 vaccination timing by integrating the theory of planned behavior and the diffusion of innovations: a cross-sectional survey in Macao, China.J Thorac Dis. 2025 May 30;17(5):2813-2826. doi: 10.21037/jtd-24-1313. Epub 2025 May 27. J Thorac Dis. 2025. PMID: 40529767 Free PMC article.
-
Prevalence and factors of COVID-19 vaccine refusal among solid cancer patients in China: an application of the health belief model.Front Public Health. 2023 Aug 3;11:1236376. doi: 10.3389/fpubh.2023.1236376. eCollection 2023. Front Public Health. 2023. PMID: 37601174 Free PMC article.
References
-
- Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP, Chaicumpa W.. COVID-19, an emerging coronavirus infection: Advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 2020;16(6):1–10. doi:10.1080/21645515.2020.1735227. - DOI - PMC - PubMed
-
- Zhu FC, Guan XH, Li YH, Huang JY, Jiang T, Hou LH, Li JX, Yang BF, Wang L, Wang WJ. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488. doi:10.1016/S0140-6736(20)31605-6. - DOI - PMC - PubMed
-
- MedSci . Bulletin of global COVID-19 and vaccination on October 18, 2021. [accessed 2021 Oct 30]. https://www.medsci.cn/article/show_article.do?id=79a3218e95fd.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical