Decision-Making Scoring System for the Repetition of Conventional Transarterial Chemoembolization in Patients With Inoperable Hepatocellular Carcinoma
- PMID: 35905418
- PMCID: PMC10476822
- DOI: 10.14309/ctg.0000000000000506
Decision-Making Scoring System for the Repetition of Conventional Transarterial Chemoembolization in Patients With Inoperable Hepatocellular Carcinoma
Abstract
Introduction: Patients with unresectable hepatocellular carcinoma treated with conventional transarterial chemoembolization (cTACE) have heterogeneous tumor burden and liver function. Therefore, the selection of patients for repeated cTACE is challenging owing to different outcomes. This study aimed to establish a decision-making scoring system for repeated cTACE to guide further treatment.
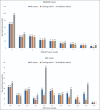
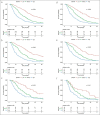
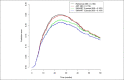
Methods: All patients with hepatocellular carcinoma who underwent cTACE between 2008 and 2019 were included and randomly assigned into training (n = 324) and validation (n = 162) cohorts. Tumor Size, number of Masses, Albumin-bilirubin score, baseline Alpha-fetoprotein level, and Response to initial cTACE session were selected to generate a "SMAART" score in the training cohort. Patients were stratified according to the SMAART score: low risk, 0-2; medium risk, 3-4; and high risk, 5-8. Prediction error curves based on the integrated Brier score and the Harrell C-index validated the SMAART scores and compared them with the Assessment for Retreatment with Transarterial chemoembolization (ART) score.
Results: The low-risk group had the longest median overall survival of 39.0 months, followed by the medium-risk and high-risk groups of 21.2 months and 10.5 months, respectively, with significant differences (P < 0.001). The validation cohort had similar results. The high-risk group had 63.1% TACE refractory cases. The Harrell C-indexes were 0.562 and 0.665 and the integrated Brier scores were 0.176 and 0.154 for ART and SMAART scores, respectively.
Discussion: The SMAART score can aid clinicians in selecting appropriate candidates for subsequent cTACE. A SMAART score of ≥5 after the first cTACE session identified patients with poor prognosis who may not benefit from additional cTACE sessions.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures

Similar articles
-
Lack of Response to Transarterial Chemoembolization for Intermediate-Stage Hepatocellular Carcinoma: Abandon or Repeat?Radiology. 2021 Mar;298(3):680-692. doi: 10.1148/radiol.2021202289. Epub 2021 Jan 19. Radiology. 2021. PMID: 33464183
-
Imaging Changes and Clinical Complications After Drug-Eluting Bead Versus Conventional Transarterial Chemoembolization for Unresectable Hepatocellular Carcinoma: Multicenter Study.AJR Am J Roentgenol. 2021 Oct;217(4):933-943. doi: 10.2214/AJR.20.24708. Epub 2020 Nov 27. AJR Am J Roentgenol. 2021. PMID: 33245680
-
Validation of Clinical Scoring Systems ART and ABCR after Transarterial Chemoembolization of Hepatocellular Carcinoma.J Vasc Interv Radiol. 2017 Jan;28(1):94-102. doi: 10.1016/j.jvir.2016.06.012. Epub 2016 Aug 23. J Vasc Interv Radiol. 2017. PMID: 27562621
-
Conventional versus drug-eluting beads chemoembolization for infiltrative hepatocellular carcinoma: a comparison of efficacy and safety.BMC Cancer. 2019 Nov 29;19(1):1162. doi: 10.1186/s12885-019-6386-6. BMC Cancer. 2019. PMID: 31783814 Free PMC article.
-
Initiative on Superselective Conventional Transarterial Chemoembolization Results (INSPIRE).Cardiovasc Intervent Radiol. 2022 Oct;45(10):1430-1440. doi: 10.1007/s00270-022-03233-9. Epub 2022 Aug 17. Cardiovasc Intervent Radiol. 2022. PMID: 35978174 Free PMC article. Review.
Cited by
-
Sultan's Score: A Novel Predictive Score to Predict Complete Response Following Drug-Eluting Bead Chemoembolization.Cureus. 2025 Jan 2;17(1):e76822. doi: 10.7759/cureus.76822. eCollection 2025 Jan. Cureus. 2025. PMID: 39758864 Free PMC article.
-
Prognostic Value of Myosteatosis and Albumin-Bilirubin Grade for Survival in Hepatocellular Carcinoma Post Chemoembolization.Cancers (Basel). 2024 Oct 17;16(20):3503. doi: 10.3390/cancers16203503. Cancers (Basel). 2024. PMID: 39456597 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. - PubMed
-
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358–80. - PubMed
-
- Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164–71. - PubMed
-
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003;37:429–42. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

