Harnessing cortical plasticity via gabapentinoid administration promotes recovery after stroke
- PMID: 35905466
- PMCID: PMC9890504
- DOI: 10.1093/brain/awac103
Harnessing cortical plasticity via gabapentinoid administration promotes recovery after stroke
Abstract
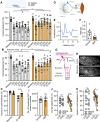
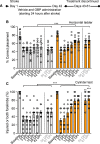
Stroke causes devastating sensory-motor deficits and long-term disability due to disruption of descending motor pathways. Restoration of these functions enables independent living and therefore represents a high priority for those afflicted by stroke. Here, we report that daily administration of gabapentin, a clinically approved drug already used to treat various neurological disorders, promotes structural and functional plasticity of the corticospinal pathway after photothrombotic cortical stroke in adult mice. We found that gabapentin administration had no effects on vascular occlusion, haemodynamic changes nor survival of corticospinal neurons within the ipsilateral sensory-motor cortex in the acute stages of stroke. Instead, using a combination of tract tracing, electrical stimulation and functional connectivity mapping, we demonstrated that corticospinal axons originating from the contralateral side of the brain in mice administered gabapentin extend numerous collaterals, form new synaptic contacts and better integrate within spinal circuits that control forelimb muscles. Not only does gabapentin daily administration promote neuroplasticity, but it also dampens maladaptive plasticity by reducing the excitability of spinal motor circuitry. In turn, mice administered gabapentin starting 1 h or 1 day after stroke recovered skilled upper extremity function. Functional recovery persists even after stopping the treatment at 6 weeks following a stroke. Finally, chemogenetic silencing of cortical projections originating from the contralateral side of the brain transiently abrogated recovery in mice administered gabapentin, further supporting the conclusion that gabapentin-dependent reorganization of spared cortical pathways drives functional recovery after stroke. These observations highlight the strong potential for repurposing gabapentinoids as a promising treatment strategy for stroke repair.
Keywords: corticospinal tract; functional recovery; gabapentinoids; stroke; structural plasticity.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
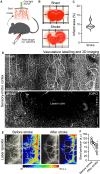
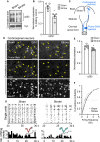
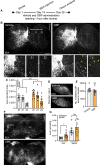
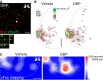
Similar articles
-
Gabapentinoid treatment promotes corticospinal plasticity and regeneration following murine spinal cord injury.J Clin Invest. 2020 Jan 2;130(1):345-358. doi: 10.1172/JCI130391. J Clin Invest. 2020. PMID: 31793909 Free PMC article.
-
Re-Establishment of Cortical Motor Output Maps and Spontaneous Functional Recovery via Spared Dorsolaterally Projecting Corticospinal Neurons after Dorsal Column Spinal Cord Injury in Adult Mice.J Neurosci. 2016 Apr 6;36(14):4080-92. doi: 10.1523/JNEUROSCI.3386-15.2016. J Neurosci. 2016. PMID: 27053214 Free PMC article.
-
Selective long-term reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury.J Comp Neurol. 2010 Mar 1;518(5):586-621. doi: 10.1002/cne.22218. J Comp Neurol. 2010. PMID: 20034062 Free PMC article.
-
Neuroplasticity of spinal cord injury and repair.Handb Clin Neurol. 2022;184:317-330. doi: 10.1016/B978-0-12-819410-2.00017-5. Handb Clin Neurol. 2022. PMID: 35034745 Review.
-
Corticospinal Motor Circuit Plasticity After Spinal Cord Injury: Harnessing Neuroplasticity to Improve Functional Outcomes.Mol Neurobiol. 2021 Nov;58(11):5494-5516. doi: 10.1007/s12035-021-02484-w. Epub 2021 Aug 3. Mol Neurobiol. 2021. PMID: 34341881 Review.
Cited by
-
Cost-effective pharmacological approaches to poststroke recovery and enhanced neuroplasticity: highlighting discoveries both old and new!Ann Med Surg (Lond). 2023 Mar 16;85(4):639-641. doi: 10.1097/MS9.0000000000000246. eCollection 2023 Apr. Ann Med Surg (Lond). 2023. PMID: 37113913 Free PMC article. No abstract available.
-
The MT1 receptor as the target of ramelteon neuroprotection in ischemic stroke.J Pineal Res. 2024 Jan;76(1):e12925. doi: 10.1111/jpi.12925. Epub 2023 Nov 20. J Pineal Res. 2024. PMID: 37986632 Free PMC article.
-
Closed-Loop Wearable Device Network of Intrinsically-Controlled, Bilateral Coordinated Functional Electrical Stimulation for Stroke.Adv Sci (Weinh). 2024 May;11(17):e2304763. doi: 10.1002/advs.202304763. Epub 2024 Mar 1. Adv Sci (Weinh). 2024. PMID: 38429890 Free PMC article.
-
Plasticity of Mouse Dorsal Root Ganglion Neurons by Innate Immune Activation Is Influenced by Electrophysiological Activity.J Neurochem. 2025 Jan;169(1):e16292. doi: 10.1111/jnc.16292. J Neurochem. 2025. PMID: 39725852 Free PMC article.
-
Sleep Disorders and Stroke: Pathophysiological Links, Clinical Implications, and Management Strategies.Med Sci (Basel). 2025 Aug 5;13(3):113. doi: 10.3390/medsci13030113. Med Sci (Basel). 2025. PMID: 40843734 Free PMC article. Review.
References
-
- Murphy TH, Corbett D. Plasticity during stroke recovery: From synapse to behaviour. Nat Rev Neurosci. 2009;10:861–872. - PubMed
-
- Ward NS, Brander F, Kelly K. Intensive upper limb neurorehabilitation in chronic stroke: Outcomes from the Queen Square programme. J Neurol Neurosurg Psychiatry. 2019;90:498–506. - PubMed
-
- Tedeschi A, Bradke F. Spatial and temporal arrangement of neuronal intrinsic and extrinsic mechanisms controlling axon regeneration. Curr Opin Neurobiol. 2017;42:118–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

