Current evidence on the use of anakinra in COVID-19
- PMID: 35905562
- PMCID: PMC9296834
- DOI: 10.1016/j.intimp.2022.109075
Current evidence on the use of anakinra in COVID-19
Abstract
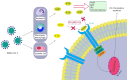
Despite the progressing knowledge in COVID-19 management, remdesivir is the only agent that got approval to inhibit viral replication. However, there are limited data about effective immunomodulatory agents to prevent cytokine release in COVID-19. Cytokine release syndrome in COVID-19 resembles secondary hemophagocytic lymphohistiocytosis, in which interleukin-1 (IL-1) plays a key role. Anakinra is the first recombinant IL-1 receptor antagonist studied for off-label use in COVID-19 treatment. This study reviews the current clinical evidence on the role of interleukin-1 in COVID-19-related cytokine storm, therapeutic effects, significant clinical concerns, and pros and cons of anakinra administration in the management of COVID-19 patients. In this review, four items are shown to be important for achieving the optimal therapeutic effects of anakinra in COVID-19 patients. These items include duration of treatment ≥ 10 days, doses ≥ 100 mg, intravenous administration, and early initiation of therapy. Also, anakinra might be more beneficial in the early stages of the disease when higher levels of cytokines are yet to be observed, which could prevent progression to severe illness and mechanical ventilation. Further studies are required to address the SARS-CoV-2 induced cytokine release syndrome and the role of anakinra in identifying ideal treatment approaches for COVID-19 patients based on their clinical status.
Keywords: Acute respiratory distress syndrome; Anakinra; COVID-19; Cytokine release syndrome; Interleukin-1; Multiple organ failure.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- WHO Coronavirus (COVID-19) Dashboard, World Heal. Organ. (n.d.). https://covid19.who.int.
-
- Symptoms of COVID-19, 2021, Centers Dis. Control Prev. (n.d.). https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html.
-
- Hasan S.S., Capstick T., Ahmed R., Kow C.S., Mazhar F., Merchant H.a., Zaidi S.T.R. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis. Expert Rev. Respir. Med. 2020;14(11):1149–1163. doi: 10.1080/17476348.2020.1804365. - DOI - PMC - PubMed
-
- de la Calle C., López-Medrano F., Pablos J.L., Lora-Tamayo J., Maestro-de la Calle G., Sánchez-Fernández M., Fernández-Ruiz M., Pérez-Jacoiste Asín M.A., Caro-Teller J.M., García-García R., Catalán M., Martínez-López J., Sevillano Á., Origüen J., Ripoll M., Juan R.S., Lalueza A., de Miguel B., Carretero O., Aguilar F., Gómez C., Paz-Artal E., Bueno H., Lumbreras C., Aguado J.M. Effectiveness of anakinra for tocilizumab-refractory severe COVID-19: a single-centre retrospective comparative study. Int. J. Infect. Dis. 2021;105:319–325. doi: 10.1016/j.ijid.2021.02.041. - DOI - PMC - PubMed
-
- EMA recommends approval for use of Kineret in adults with COVID-19, Eur. Med. Agency. (n.d.).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

