Inhibition of CSPG receptor PTPσ promotes migration of newly born neuroblasts, axonal sprouting, and recovery from stroke
- PMID: 35905716
- PMCID: PMC9677607
- DOI: 10.1016/j.celrep.2022.111137
Inhibition of CSPG receptor PTPσ promotes migration of newly born neuroblasts, axonal sprouting, and recovery from stroke
Abstract
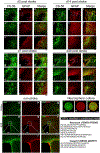
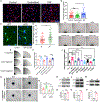
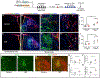
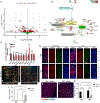
In addition to neuroprotective strategies, neuroregenerative processes could provide targets for stroke recovery. However, the upregulation of inhibitory chondroitin sulfate proteoglycans (CSPGs) impedes innate regenerative efforts. Here, we examine the regulatory role of PTPσ (a major proteoglycan receptor) in dampening post-stroke recovery. Use of a receptor modulatory peptide (ISP) or Ptprs gene deletion leads to increased neurite outgrowth and enhanced NSCs migration upon inhibitory CSPG substrates. Post-stroke ISP treatment results in increased axonal sprouting as well as neuroblast migration deeply into the lesion scar with a transcriptional signature reflective of repair. Lastly, peptide treatment post-stroke (initiated acutely or more chronically at 7 days) results in improved behavioral recovery in both motor and cognitive functions. Therefore, we propose that CSPGs induced by stroke play a predominant role in the regulation of neural repair and that blocking CSPG signaling pathways will lead to enhanced neurorepair and functional recovery in stroke.
Keywords: CP: Neuroscience; CSPGs; PTPσ; axonal regeneration and sprouting; functional recovery; neurogenesis; proximal MCAo; stroke.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests F.L., J.S., and Y.L. are listed as inventors in a patent application that has been submitted by CWRU that is based partially on these results. J.S. is an advisor to NervGen, a startup pharmaceutical company that has licensed from CWRU an issued patent (#9937242) covering the ISP peptide.
Figures



Similar articles
-
Perturbing chondroitin sulfate proteoglycan signaling through LAR and PTPσ receptors promotes a beneficial inflammatory response following spinal cord injury.J Neuroinflammation. 2018 Mar 20;15(1):90. doi: 10.1186/s12974-018-1128-2. J Neuroinflammation. 2018. PMID: 29558941 Free PMC article.
-
Suppressing CSPG/LAR/PTPσ Axis Facilitates Neuronal Replacement and Synaptogenesis by Human Neural Precursor Grafts and Improves Recovery after Spinal Cord Injury.J Neurosci. 2022 Apr 13;42(15):3096-3121. doi: 10.1523/JNEUROSCI.2177-21.2022. Epub 2022 Mar 7. J Neurosci. 2022. PMID: 35256527 Free PMC article.
-
Chondroitin sulfate proteoglycans inhibit oligodendrocyte myelination through PTPσ.Exp Neurol. 2013 Sep;247:113-21. doi: 10.1016/j.expneurol.2013.04.003. Epub 2013 Apr 12. Exp Neurol. 2013. PMID: 23588220
-
Regulation of autophagy by inhibitory CSPG interactions with receptor PTPσ and its impact on plasticity and regeneration after spinal cord injury.Exp Neurol. 2020 Jun;328:113276. doi: 10.1016/j.expneurol.2020.113276. Epub 2020 Mar 4. Exp Neurol. 2020. PMID: 32145250 Free PMC article. Review.
-
Protein tyrosine phosphatase σ in proteoglycan-mediated neural regeneration regulation.Mol Neurobiol. 2013 Feb;47(1):220-7. doi: 10.1007/s12035-012-8346-x. Epub 2012 Sep 7. Mol Neurobiol. 2013. PMID: 22956273 Review.
Cited by
-
Inhibition of CSPG-PTPσ Activates Autophagy Flux and Lysosome Fusion, Aids Axon and Synaptic Reorganization in Spinal Cord Injury.Mol Neurobiol. 2025 Jan;62(1):773-785. doi: 10.1007/s12035-024-04304-3. Epub 2024 Jun 20. Mol Neurobiol. 2025. PMID: 38900368
-
The choroid plexus maintains ventricle volume and adult subventricular zone neuroblast pool, which facilitates post-stroke neurogenesis.bioRxiv [Preprint]. 2024 Jan 23:2024.01.22.575277. doi: 10.1101/2024.01.22.575277. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2024 Jul 9;121(28):e2400213121. doi: 10.1073/pnas.2400213121. PMID: 38328050 Free PMC article. Updated. Preprint.
-
Changing genes, cells and networks to reprogram the brain after stroke.Nat Neurosci. 2025 Jun;28(6):1130-1145. doi: 10.1038/s41593-025-01981-8. Epub 2025 Jun 2. Nat Neurosci. 2025. PMID: 40456908 Review.
-
Identification of the growth cone as a probe and driver of neuronal migration in the injured brain.Nat Commun. 2024 Mar 9;15(1):1877. doi: 10.1038/s41467-024-45825-8. Nat Commun. 2024. PMID: 38461182 Free PMC article.
-
Small extracellular vesicles derived from cerebral endothelial cells with elevated microRNA 27a promote ischemic stroke recovery.Neural Regen Res. 2025 Jan 1;20(1):224-233. doi: 10.4103/NRR.NRR-D-22-01292. Epub 2024 Mar 1. Neural Regen Res. 2025. PMID: 38767487 Free PMC article.
References
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, and Lindvall O (2002). Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med 8, 963–970. - PubMed
-
- Bartus K, James ND, Didangelos A, Bosch KD, Verhaagen J, Yáñez-Muñoz RJ, Rogers JH, Schneider BL, Muir EM, and Bradbury EJ (2014). Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J. Neurosci 34, 4822–4836. 10.1523/JNEUROSCI.4369-13.2014. - DOI - PMC - PubMed
-
- Bunin A, Sisirak V, Ghosh HS, Grajkowska LT, Hou ZE, Miron M, Yang C, Ceribelli M, Uetani N, Chaperot L, et al. (2015). Protein tyrosine phosphatase PTPRS is an inhibitory receptor on human and murine plasmacytoid dendritic cells. Immunity 43, 277–288. 10.1016/j.immuni.2015.07.009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

