Dynamic control of visually guided locomotion through corticosubthalamic projections
- PMID: 35905719
- PMCID: PMC9395210
- DOI: 10.1016/j.celrep.2022.111139
Dynamic control of visually guided locomotion through corticosubthalamic projections
Abstract
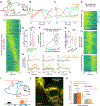
Goal-directed locomotion requires control signals that propagate from higher order areas to regulate spinal mechanisms. The corticosubthalamic hyperdirect pathway offers a short route for cortical information to reach locomotor centers in the brainstem. We developed a task in which head-fixed mice run to a visual landmark and then stop and wait to collect the reward and examined the role of secondary motor cortex (M2) projections to the subthalamic nucleus (STN) in controlling locomotion. Our behavioral modeling, calcium imaging, and optogenetics manipulation results suggest that the M2-STN pathway can be recruited during visually guided locomotion to rapidly and precisely control the pedunculopontine nucleus (PPN) of the mesencephalic locomotor region through the basal ganglia. By capturing the physiological dynamics through a feedback control model and analyzing neuronal signals in M2, PPN, and STN, we find that the corticosubthalamic projections potentially control PPN activity by differentiating an M2 error signal to ensure fast input-output dynamics.
Keywords: CP: Neuroscience; controller; dynamical system; hyperdirect pathway; landmark; locomotion; mesencephalic locomotor region; pedunculopontine nucleus; secondary motor cortex; stopping; subthalamic nucleus; visually guided.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Similar articles
-
Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction.Neuroscience. 2003;119(1):293-308. doi: 10.1016/s0306-4522(03)00095-2. Neuroscience. 2003. PMID: 12763089
-
The network of causal interactions for beta oscillations in the pedunculopontine nucleus, primary motor cortex, and subthalamic nucleus of walking parkinsonian rats.Exp Neurol. 2016 Aug;282:27-36. doi: 10.1016/j.expneurol.2016.05.007. Epub 2016 May 6. Exp Neurol. 2016. PMID: 27163550
-
Model-based deconstruction of cortical evoked potentials generated by subthalamic nucleus deep brain stimulation.J Neurophysiol. 2018 Aug 1;120(2):662-680. doi: 10.1152/jn.00862.2017. Epub 2018 Apr 25. J Neurophysiol. 2018. PMID: 29694280 Free PMC article.
-
The basal ganglia and the locomotor regions.Brain Res. 1986 Mar;396(1):47-63. Brain Res. 1986. PMID: 2871904 Review.
-
Rethinking the Pedunculopontine Nucleus: From Cellular Organization to Function.Neuron. 2017 Apr 5;94(1):7-18. doi: 10.1016/j.neuron.2017.02.027. Neuron. 2017. PMID: 28384477 Review.
Cited by
-
Effects of chronic stress on cognitive function - From neurobiology to intervention.Neurobiol Stress. 2024 Sep 2;33:100670. doi: 10.1016/j.ynstr.2024.100670. eCollection 2024 Nov. Neurobiol Stress. 2024. PMID: 39295772 Free PMC article. Review.
-
Bio-Inspired Design of Superconducting Spiking Neuron and Synapse.Nanomaterials (Basel). 2023 Jul 19;13(14):2101. doi: 10.3390/nano13142101. Nanomaterials (Basel). 2023. PMID: 37513112 Free PMC article.
-
Adaptation of sequential action benefits from timing variability related to lateral basal ganglia circuitry.iScience. 2024 Feb 20;27(3):109274. doi: 10.1016/j.isci.2024.109274. eCollection 2024 Mar 15. iScience. 2024. PMID: 38496293 Free PMC article.
-
Cell and circuit complexity of the external globus pallidus.Nat Neurosci. 2023 Jul;26(7):1147-1159. doi: 10.1038/s41593-023-01368-7. Epub 2023 Jun 19. Nat Neurosci. 2023. PMID: 37336974 Free PMC article. Review.
-
Algebraic approach for subspace decomposition and clustering of neural activity.STAR Protoc. 2022 Nov 11;3(4):101841. doi: 10.1016/j.xpro.2022.101841. eCollection 2022 Dec 16. STAR Protoc. 2022. PMID: 36386884 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

