Rapid GPR183-mediated recruitment of eosinophils to the lung after Mycobacterium tuberculosis infection
- PMID: 35905725
- PMCID: PMC9460869
- DOI: 10.1016/j.celrep.2022.111144
Rapid GPR183-mediated recruitment of eosinophils to the lung after Mycobacterium tuberculosis infection
Abstract
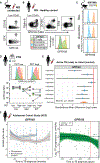
Influx of eosinophils into the lungs is typically associated with type II responses during allergy and fungal and parasitic infections. However, we previously reported that eosinophils accumulate in lung lesions during type I inflammatory responses to Mycobacterium tuberculosis (Mtb) in humans, macaques, and mice, in which they support host resistance. Here we show eosinophils migrate into the lungs of macaques and mice as early as one week after Mtb exposure. In mice this influx is CCR3 independent and instead requires cell-intrinsic expression of the oxysterol receptor GPR183, which is highly expressed on human and macaque eosinophils. Murine eosinophils interact directly with bacilli-laden alveolar macrophages, which upregulate the oxysterol-synthesizing enzyme Ch25h, and eosinophil recruitment is impaired in Ch25h-deficient mice. Our findings show that eosinophils are among the earliest cells from circulation to sense and respond to Mtb infection of alveolar macrophages and reveal a role for GPR183 in the migration of eosinophils into lung tissue.
Keywords: CCR3; CP: Immunology; CP: Microbiology; Ch25h; GPR183; Mycobacterium tuberculosis; NHP; alveolar macrophages; bacterial infection; eosinophils; eotaxin; granulocytes; lung; neutrophils; nonhuman primate; oxysterols; rhesus macaque.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Baptista AP, Gola A, Huang Y, Milanez-Almeida P, Torabi-Parizi P, Urban JF Jr., Shapiro VS, Gerner MY, and Germain RN (2019). The chemoattractant receptor Ebi2 drives intranodal naive CD4(+) T cell peripheralization to promote effective adaptive immunity. Immunity 50, 1188–1201.e6. 10.1016/j.immuni.2019.04.001. - DOI - PubMed
-
- Bartlett S, Gemiarto AT, Ngo MD, Sajiir H, Hailu S, Sinha R, Foo CX, Kleynhans L, Tshivhula H, Webber T, et al. (2020). GPR183 regulates interferons, autophagy, and bacterial growth during Mycobacterium tuberculosis infection and is associated with TB disease severity. Front. Immunol 11, 601534. 10.3389/fimmu.2020.601534. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

