Safety and immunogenicity of inactivated SARS-CoV-2 vaccines in people with gastrointestinal cancer
- PMID: 35905950
- PMCID: PMC9316719
- DOI: 10.1016/j.ijid.2022.07.050
Safety and immunogenicity of inactivated SARS-CoV-2 vaccines in people with gastrointestinal cancer
Abstract
Objectives: This study aimed to evaluate the safety and immunogenicity of inactivated COVID-19 vaccines in patients with gastrointestinal cancer (GI) cancer. The role of memory B cells (MBCs) in the humoral response to COVID-19 vaccination was also investigated.
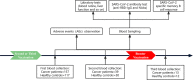
Methods: In this prospective observational study, GI cancer patients and healthy individuals who had received 2 doses of inactivated COVID-19 vaccines were included. The data regarding adverse effects, serum anti-receptor binding domain (RBD)-IgG, neutralizing antibodies (NAbs), and frequencies of MBCs were collected prospectively.
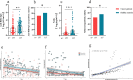
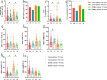
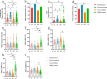
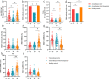
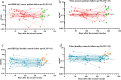
Results: The inactivated COVID-19 vaccines were safe and well tolerated. Serum anti-RBG-IgG and NAbs were lower for cancer patients. Old age, high ASA score, and receiving active chemotherapy were risk factors for lower antibody titers. The frequencies of activated and resting MBCs decreased in (17.45% vs 38.11%, P = 0.002; 16.98% vs 34.13%, P = 0.023), while the frequencies of intermediate and atypical MBCs increased in cancer patients (40.06% vs 19.87%, P = 0.010; 25.47% vs 16.61%, P = 0.025). The serum antibody titer decreased gradually during follow-up but increased when a booster vaccine was given.
Conclusion: The inactivated COVID-19 vaccines were well tolerated in patients with GI cancer but with lower immunogenicity. The subpopulations of MBCs were disordered in cancer patients, and a booster vaccine may be prioritized for them.
Keywords: COVID-19; Inactivated vaccine; Memory B cells.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors have no conflicts of interest to declare.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

