Cross-species transcriptome analysis for early detection and specific therapeutic targeting of human lupus nephritis
- PMID: 35906002
- PMCID: PMC9484391
- DOI: 10.1136/annrheumdis-2021-222069
Cross-species transcriptome analysis for early detection and specific therapeutic targeting of human lupus nephritis
Abstract
Objectives: Patients with lupus nephritis (LN) are in urgent need for early diagnosis and therapeutic interventions targeting aberrant molecular pathways enriched in affected kidneys.
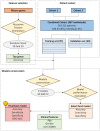
Methods: We used mRNA-sequencing in effector (spleen) and target (kidneys, brain) tissues from lupus and control mice at sequential time points, and in the blood from 367 individuals (261 systemic lupus erythematosus (SLE) patients and 106 healthy individuals). Comparative cross-tissue and cross-species analyses were performed. The human dataset was split into training and validation sets and machine learning was applied to build LN predictive models.
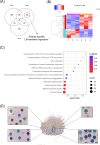
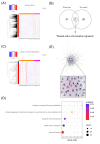
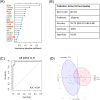
Results: In murine SLE, we defined a kidney-specific molecular signature, as well as a molecular signature that underlies transition from preclinical to overt disease and encompasses pathways linked to metabolism, innate immune system and neutrophil degranulation. The murine kidney transcriptome partially mirrors the blood transcriptome of patients with LN with 11 key transcription factors regulating the cross-species active LN molecular signature. Integrated protein-to-protein interaction and drug prediction analyses identified the kinases TRRAP, AKT2, CDK16 and SCYL1 as putative targets of these factors and capable of reversing the LN signature. Using murine kidney-specific genes as disease predictors and machine-learning training of the human RNA-sequencing dataset, we developed and validated a peripheral blood-based algorithm that discriminates LN patients from normal individuals (based on 18 genes) and non-LN SLE patients (based on 20 genes) with excellent sensitivity and specificity (area under the curve range from 0.80 to 0.99).
Conclusions: Machine-learning analysis of a large whole blood RNA-sequencing dataset of SLE patients using human orthologs of mouse kidney-specific genes can be used for early, non-invasive diagnosis and therapeutic targeting of LN. The kidney-specific gene predictors may facilitate prevention and early intervention trials.
Keywords: Autoimmune Diseases; Autoimmunity; Lupus Nephritis; Systemic Lupus Erythematosus; Therapeutics.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: ED is an employee of GSK. His contribution was performed before he joined GSK.
Figures

References
-
- Merrill JT, Neuwelt CM, Wallace DJ, et al. . Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum : 2010;62:222–33. 10.1002/art.27233 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

