Replication of alphaviruses requires a pseudoknot that involves the poly(A) tail
- PMID: 35906005
- PMCID: PMC9479738
- DOI: 10.1261/rna.079243.122
Replication of alphaviruses requires a pseudoknot that involves the poly(A) tail
Abstract
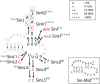
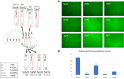
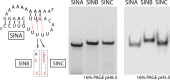
Alphaviruses, such as the Sindbis virus and the Chikungunya virus, are RNA viruses with a positive sense single-stranded RNA genome that infect various vertebrates, including humans. A conserved sequence element (CSE) of ∼19 nt in the 3' noncoding region is important for replication. Despite extensive mutational analysis of the CSE, no comprehensive model of this element exists to date. Here, it is shown that the CSE can form an RNA pseudoknot with part of the poly(A) tail and is similar to the human telomerase pseudoknot with which it shares 17 nt. Mutants that alter the stability of the pseudoknot were investigated in the context of a replicon of the Sindbis virus and by native gel electrophoresis. These studies reveal that the pseudoknot is required for virus replication and is stabilized by UAU base triples. The new model is discussed in relation to previous data on Sindbis virus mutants and revertants lacking (part of) the CSE.
Keywords: alphavirus; base triple; poly(A) tail; pseudoknot.
© 2022 Olsthoorn; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures







Similar articles
-
Role of an RNA pseudoknot involving the polyA tail in replication of Pepino mosaic potexvirus and related plant viruses.Sci Rep. 2022 Jul 7;12(1):11532. doi: 10.1038/s41598-022-15598-5. Sci Rep. 2022. PMID: 35798958 Free PMC article.
-
In vivo addition of poly(A) tail and AU-rich sequences to the 3' terminus of the Sindbis virus RNA genome: a novel 3'-end repair pathway.J Virol. 1999 Mar;73(3):2410-9. doi: 10.1128/JVI.73.3.2410-2419.1999. J Virol. 1999. PMID: 9971825 Free PMC article.
-
Requirements at the 3' end of the sindbis virus genome for efficient synthesis of minus-strand RNA.J Virol. 2005 Apr;79(8):4630-9. doi: 10.1128/JVI.79.8.4630-4639.2005. J Virol. 2005. PMID: 15795249 Free PMC article.
-
Alphavirus vectors: development and potential therapeutic applications.Expert Opin Biol Ther. 2001 Mar;1(2):177-91. doi: 10.1517/14712598.1.2.177. Expert Opin Biol Ther. 2001. PMID: 11727528 Review.
-
Dancing with the Devil: A Review of the Importance of Host RNA-Binding Proteins to Alphaviral RNAs during Infection.Viruses. 2023 Jan 5;15(1):164. doi: 10.3390/v15010164. Viruses. 2023. PMID: 36680204 Free PMC article. Review.
Cited by
-
Safety and immunogenicity of an optimized self-replicating RNA platform for low dose or single dose vaccine applications: a randomized, open label Phase I study in healthy volunteers.Nat Commun. 2025 Jan 7;16(1):456. doi: 10.1038/s41467-025-55843-9. Nat Commun. 2025. PMID: 39774967 Free PMC article. Clinical Trial.
-
Mapping the structural landscape of the yeast Ty3 retrotransposon RNA genome.Nucleic Acids Res. 2024 Sep 9;52(16):9821-9837. doi: 10.1093/nar/gkae494. Nucleic Acids Res. 2024. PMID: 38864374 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
