The new generation of messenger RNA (mRNA) vaccines against influenza
- PMID: 35906174
- PMCID: PMC9315338
- DOI: 10.1016/j.eimce.2022.07.006
The new generation of messenger RNA (mRNA) vaccines against influenza
Abstract
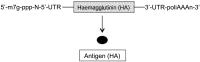
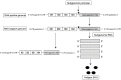
Today there are multiple types of flu vaccines. The emergence of nucleic acid technology used in vaccines against SARS-CoV-2 suggests its future application against this infection. Against influenza, two types of vaccines have been developed based on messenger RNA (mRNA): conventional or non-replicative and self-amplifying or replicative (auRNA), both included in lipid nanoparticles. Animal studies carried out with the former have shown their strong capacity to induce Th-1 antibodies and cellular immunity against influenza haemagglutinin (HA) with few side effects. Human trials have shown 87% seroconversion and 100% seroprotection. The auRNA vaccines have obtained similar results in animals but at a concentration 64 times lower than the conventional one. Vaccines based on mRNA platforms meet the WHO requirements for next generation influenza vaccines.
En la actualidad existen múltiples tipos de vacunas frente a la gripe. La irrupción de la tecnología de ácidos nucleicos utilizada en las vacunas frente al SARS-CoV-2 hace pensar en su aplicación futura frente a esta infección. Frente a la gripe se han desarrollado dos tipos de vacunas basadas en el ARN mensajero (ARNm): las convencionales o no replicativas y las autoamplificables o replicativas (auARNm), ambas incluidas en nanopartículas lipídicas. Los estudios en animales realizados con las primeras han mostrado su intensa capacidad para inducir anticuerpos e inmunidad celular Th-1 frente a la hemaglutinina (HA) gripal con escasos efectos secundarios. Los ensayos en humanos han mostrado una seroconversión del 87% y una seroprotección del 100%. Las vacunas auARNm han obtenido resultados en animales semejantes pero a una concentración 64 veces inferior a la convencional. Las vacunas basadas en las plataformas de ARNm cumplen los requisitos establecidos por la OMS para vacunas de gripe de la generación siguiente.
Keywords: Eficacia vacunal; Flu; Gripe; Messenger RNA vaccines; Vaccine efficacy; Vacunas ARN mensajero.
Copyright © 2021 Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica. Published by Elsevier España, S.L.U. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous