Radiotherapy combined with immunotherapy: the dawn of cancer treatment
- PMID: 35906199
- PMCID: PMC9338328
- DOI: 10.1038/s41392-022-01102-y
Radiotherapy combined with immunotherapy: the dawn of cancer treatment
Abstract
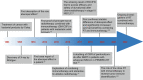
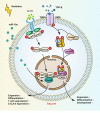
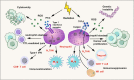
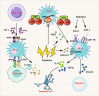
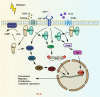
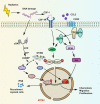
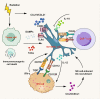
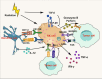
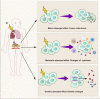
Radiotherapy (RT) is delivered for purposes of local control, but can also exert systemic effect on remote and non-irradiated tumor deposits, which is called abscopal effect. The view of RT as a simple local treatment has dramatically changed in recent years, and it is now widely accepted that RT can provoke a systemic immune response which gives a strong rationale for the combination of RT and immunotherapy (iRT). Nevertheless, several points remain to be addressed such as the interaction of RT and immune system, the identification of the best schedules for combination with immunotherapy (IO), the expansion of abscopal effect and the mechanism to amplify iRT. To answer these crucial questions, we roundly summarize underlying rationale showing the whole immune landscape in RT and clinical trials to attempt to identify the best schedules of iRT. In consideration of the rarity of abscopal effect, we propose that the occurrence of abscopal effect induced by radiation can be promoted to 100% in view of molecular and genetic level. Furthermore, the "radscopal effect" which refers to using low-dose radiation to reprogram the tumor microenvironment may amplify the occurrence of abscopal effect and overcome the resistance of iRT. Taken together, RT could be regarded as a trigger of systemic antitumor immune response, and with the help of IO can be used as a radical and systemic treatment and be added into current standard regimen of patients with metastatic cancer.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

