Hypoxia aggravates ferroptosis in RPE cells by promoting the Fenton reaction
- PMID: 35906211
- PMCID: PMC9338085
- DOI: 10.1038/s41419-022-05121-z
Hypoxia aggravates ferroptosis in RPE cells by promoting the Fenton reaction
Abstract
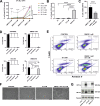
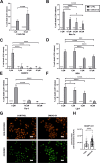
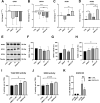
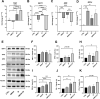
Oxidative stress and hypoxia in the retinal pigment epithelium (RPE) have long been considered major risk factors in the pathophysiology of age-related macular degeneration (AMD), but systematic investigation of the interplay between these two risk factors was lacking. For this purpose, we treated a human RPE cell line (ARPE-19) with sodium iodate (SI), an oxidative stress agent, together with dimethyloxalylglycine (DMOG) which leads to stabilization of hypoxia-inducible factors (HIFs), key regulators of cellular adaptation to hypoxic conditions. We found that HIF stabilization aggravated oxidative stress-induced cell death by SI and iron-dependent ferroptosis was identified as the main cell death mechanism. Ferroptotic cell death depends on the Fenton reaction where H2O2 and iron react to generate hydroxyl radicals which trigger lipid peroxidation. Our findings clearly provide evidence for superoxide dismutase (SOD) driven H2O2 production fostering the Fenton reaction as indicated by triggered SOD activity upon DMOG + SI treatment as well as by reduced cell death levels upon SOD2 knockdown. In addition, iron transporters involved in non-transferrin-bound Fe2+ import as well as intracellular iron levels were also upregulated. Consequently, chelation of Fe2+ by 2'2-Bipyridyl completely rescued cells. Taken together, we show for the first time that HIF stabilization under oxidative stress conditions aggravates ferroptotic cell death in RPE cells. Thus, our study provides a novel link between hypoxia, oxidative stress and iron metabolism in AMD pathophysiology. Since iron accumulation and altered iron metabolism are characteristic features of AMD retinas and RPE cells, our cell culture model is suitable for high-throughput screening of new treatment approaches against AMD.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Soundara Pandi SP, Ratnayaka JA, Lotery AJ, Teeling JL. Progress in developing rodent models of age-related macular degeneration (AMD). Exp Eye Res. 2021;203. 10.1016/j.exer.2020.108404. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

