TOB is an effector of the hippocampus-mediated acute stress response
- PMID: 35906220
- PMCID: PMC9338090
- DOI: 10.1038/s41398-022-02078-7
TOB is an effector of the hippocampus-mediated acute stress response
Abstract
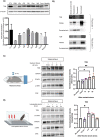
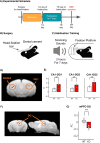
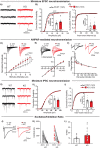
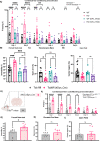
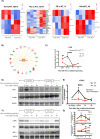
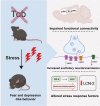
Stress affects behavior and involves critical dynamic changes at multiple levels ranging from molecular pathways to neural circuits and behavior. Abnormalities at any of these levels lead to decreased stress resilience and pathological behavior. However, temporal modulation of molecular pathways underlying stress response remains poorly understood. Transducer of ErbB2.1, known as TOB, is involved in different physiological functions, including cellular stress and immediate response to stimulation. In this study, we investigated the role of TOB in psychological stress machinery at molecular, neural circuit, and behavioral levels. Interestingly, TOB protein levels increased after mice were exposed to acute stress. At the neural circuit level, functional magnetic resonance imaging (fMRI) suggested that intra-hippocampal and hippocampal-prefrontal connectivity were dysregulated in Tob knockout (Tob-KO) mice. Electrophysiological recordings in hippocampal slices showed increased postsynaptic AMPAR-mediated neurotransmission, accompanied by decreased GABA neurotransmission and subsequently altered Excitatory/Inhibitory balance after Tob deletion. At the behavioral level, Tob-KO mice show abnormal, hippocampus-dependent, contextual fear conditioning and extinction, and depression-like behaviors. On the other hand, increased anxiety observed in Tob-KO mice is hippocampus-independent. At the molecular level, we observed changes in factors involved in stress response like decreased stress-induced LCN2 expression and ERK phosphorylation, as well as increased MKP-1 expression. This study introduces TOB as an important modulator in the hippocampal stress signaling machinery. In summary, we reveal a molecular pathway and neural circuit mechanism by which Tob deletion contributes to expression of pathological stress-related behavior.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Alteration of synaptic transmission in the hippocampal-mPFC pathway during extinction trials of context-dependent fear memory in juvenile rat stress models.Synapse. 2009 Sep;63(9):805-13. doi: 10.1002/syn.20657. Synapse. 2009. PMID: 19504621
-
Prefrontal α7nAChR Signaling Differentially Modulates Afferent Drive and Trace Fear Conditioning Behavior in Adolescent and Adult Rats.J Neurosci. 2021 Mar 3;41(9):1908-1916. doi: 10.1523/JNEUROSCI.1941-20.2020. Epub 2021 Jan 21. J Neurosci. 2021. PMID: 33478990 Free PMC article.
-
Early stress exposure impairs synaptic potentiation in the rat medial prefrontal cortex underlying contextual fear extinction.Neuroscience. 2010 Sep 15;169(4):1705-14. doi: 10.1016/j.neuroscience.2010.06.035. Epub 2010 Jun 25. Neuroscience. 2010. PMID: 20600655
-
Rab3B protein is required for long-term depression of hippocampal inhibitory synapses and for normal reversal learning.Proc Natl Acad Sci U S A. 2011 Aug 23;108(34):14300-5. doi: 10.1073/pnas.1112237108. Epub 2011 Aug 15. Proc Natl Acad Sci U S A. 2011. PMID: 21844341 Free PMC article.
-
Neurogenic Interventions for Fear Memory via Modulation of the Hippocampal Function and Neural Circuits.Int J Mol Sci. 2022 Mar 25;23(7):3582. doi: 10.3390/ijms23073582. Int J Mol Sci. 2022. PMID: 35408943 Free PMC article. Review.
Cited by
-
Optogenetic activation of dorsal raphe serotonin neurons induces brain-wide activation.Nat Commun. 2024 May 16;15(1):4152. doi: 10.1038/s41467-024-48489-6. Nat Commun. 2024. PMID: 38755120 Free PMC article.
-
The shared genetic architecture of suicidal behaviour and psychiatric disorders: A genomic structural equation modelling study.Front Genet. 2023 Mar 7;14:1083969. doi: 10.3389/fgene.2023.1083969. eCollection 2023. Front Genet. 2023. PMID: 36959830 Free PMC article.
-
Tob Regulates the Timing of Sleep Onset at Night in Drosophila.J Neurosci. 2024 May 1;44(18):e0389232024. doi: 10.1523/JNEUROSCI.0389-23.2024. J Neurosci. 2024. PMID: 38485259 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

