Functional neuronal circuitry and oscillatory dynamics in human brain organoids
- PMID: 35906223
- PMCID: PMC9338020
- DOI: 10.1038/s41467-022-32115-4
Functional neuronal circuitry and oscillatory dynamics in human brain organoids
Abstract
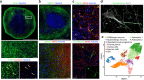
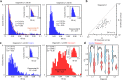
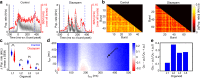
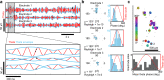
Human brain organoids replicate much of the cellular diversity and developmental anatomy of the human brain. However, the physiology of neuronal circuits within organoids remains under-explored. With high-density CMOS microelectrode arrays and shank electrodes, we captured spontaneous extracellular activity from brain organoids derived from human induced pluripotent stem cells. We inferred functional connectivity from spike timing, revealing a large number of weak connections within a skeleton of significantly fewer strong connections. A benzodiazepine increased the uniformity of firing patterns and decreased the relative fraction of weakly connected edges. Our analysis of the local field potential demonstrate that brain organoids contain neuronal assemblies of sufficient size and functional connectivity to co-activate and generate field potentials from their collective transmembrane currents that phase-lock to spiking activity. These results point to the potential of brain organoids for the study of neuropsychiatric diseases, drug action, and the effects of external stimuli upon neuronal networks.
© 2022. The Author(s).
Conflict of interest statement
K.S.K. is on the SAB of Herophilus. All other authors declare no competing interests.
Figures





Similar articles
-
Recording and Analyzing Multimodal Large-Scale Neuronal Ensemble Dynamics on CMOS-Integrated High-Density Microelectrode Array.J Vis Exp. 2024 Mar 8;(205). doi: 10.3791/66473. J Vis Exp. 2024. PMID: 38526084
-
MEA-NAP: A flexible network analysis pipeline for neuronal 2D and 3D organoid multielectrode recordings.Cell Rep Methods. 2024 Nov 18;4(11):100901. doi: 10.1016/j.crmeth.2024.100901. Epub 2024 Nov 8. Cell Rep Methods. 2024. PMID: 39520988 Free PMC article.
-
Non-Invasive and Long-Term Electrophysiological Monitoring Sensors for Cerebral Organoids Differentiation.Biosensors (Basel). 2025 Mar 7;15(3):173. doi: 10.3390/bios15030173. Biosensors (Basel). 2025. PMID: 40136970 Free PMC article.
-
Functional Characterization of Human Pluripotent Stem Cell-Derived Models of the Brain with Microelectrode Arrays.Cells. 2021 Dec 29;11(1):106. doi: 10.3390/cells11010106. Cells. 2021. PMID: 35011667 Free PMC article. Review.
-
Present and Future Modeling of Human Psychiatric Connectopathies With Brain Organoids.Biol Psychiatry. 2023 Apr 1;93(7):606-615. doi: 10.1016/j.biopsych.2022.12.017. Epub 2022 Dec 23. Biol Psychiatry. 2023. PMID: 36759258 Free PMC article. Review.
Cited by
-
Research progress of brain organoids in the field of diabetes.Mol Brain. 2024 Aug 6;17(1):53. doi: 10.1186/s13041-024-01123-4. Mol Brain. 2024. PMID: 39107846 Free PMC article. Review.
-
An Optimized Workflow to Generate and Characterize iPSC-Derived Motor Neuron (MN) Spheroids.Cells. 2023 Feb 8;12(4):545. doi: 10.3390/cells12040545. Cells. 2023. PMID: 36831212 Free PMC article.
-
Why brain organoids are not conscious yet.Patterns (N Y). 2024 Jun 24;5(8):101011. doi: 10.1016/j.patter.2024.101011. eCollection 2024 Aug 9. Patterns (N Y). 2024. PMID: 39233695 Free PMC article. Review.
-
Assembloid learning: opportunities and challenges for personalized approaches to brain functioning in health and disease.Front Artif Intell. 2024 Apr 19;7:1385871. doi: 10.3389/frai.2024.1385871. eCollection 2024. Front Artif Intell. 2024. PMID: 38708094 Free PMC article. No abstract available.
-
Advances in large-scale electrophysiology with high-density microelectrode arrays.Lab Chip. 2025 Aug 28. doi: 10.1039/d5lc00058k. Online ahead of print. Lab Chip. 2025. PMID: 40878213 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

