Vaccination with Mycoplasma pneumoniae membrane lipoproteins induces IL-17A driven neutrophilia that mediates Vaccine-Enhanced Disease
- PMID: 35906257
- PMCID: PMC9336141
- DOI: 10.1038/s41541-022-00513-w
Vaccination with Mycoplasma pneumoniae membrane lipoproteins induces IL-17A driven neutrophilia that mediates Vaccine-Enhanced Disease
Abstract
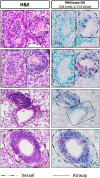
Bacterial lipoproteins are an often-underappreciated class of microbe-associated molecular patterns with potent immunomodulatory activity. We previously reported that vaccination of BALB/c mice with Mycoplasma pneumoniae (Mp) lipid-associated membrane proteins (LAMPs) resulted in lipoprotein-dependent vaccine enhanced disease after challenge with virulent Mp, though the immune responses underpinning this phenomenon remain poorly understood. Herein, we report that lipoprotein-induced VED in a mouse model is associated with elevated inflammatory cytokines TNF-α, IL-1β, IL-6, IL-17A, and KC in lung lavage fluid and with suppurative pneumonia marked by exuberant neutrophilia in the pulmonary parenchyma. Whole-lung-digest flow cytometry and RNAScope analysis identified multiple cellular sources for IL-17A, and the numbers of IL-17A producing cells were increased in LAMPs-vaccinated/Mp-challenged animals compared to controls. Specific IL-17A or neutrophil depletion reduced disease severity in our VED model-indicating that Mp lipoproteins induce VED in an IL-17A-dependent manner and through exuberant neutrophil recruitment. IL-17A neutralization reduced levels of TNF-α, IL-1β, IL-6, and KC, indicating that IL-17A preceded other inflammatory cytokines. Surprisingly, we found that IL-17A neutralization impaired bacterial clearance, while neutrophil depletion improved it-indicating that, while IL-17A appears to confer both maladaptive and protective responses, neutrophils play an entirely maladaptive role in VED. Given that lipoproteins are found in virtually all bacteria, the potential for lipoprotein-mediated maladaptive inflammatory responses should be taken into consideration when developing vaccines against bacterial pathogens.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Enhanced insights into the neutrophil-driven immune mechanisms during Mycoplasma pneumoniae infection.Heliyon. 2024 Oct 4;10(21):e38950. doi: 10.1016/j.heliyon.2024.e38950. eCollection 2024 Nov 15. Heliyon. 2024. PMID: 39524902 Free PMC article. Review.
-
Neutrophil-Mediated Lung Injury Both via TLR2-Dependent Production of IL-1α and IL-12 p40, and TLR2-Independent CARDS Toxin after Mycoplasma pneumoniae Infection in Mice.Microbiol Spectr. 2021 Dec 22;9(3):e0158821. doi: 10.1128/spectrum.01588-21. Epub 2021 Dec 22. Microbiol Spectr. 2021. PMID: 34937175 Free PMC article.
-
Lipid moieties of Mycoplasma pneumoniae lipoproteins are the causative factor of vaccine-enhanced disease.NPJ Vaccines. 2020 Apr 8;5(1):31. doi: 10.1038/s41541-020-0181-x. eCollection 2020. NPJ Vaccines. 2020. PMID: 32284882 Free PMC article.
-
Vaccination using inactivated Mycoplasma pneumoniae induces detrimental infiltration of neutrophils after subsequent infection in mice.Vaccine. 2020 Jul 6;38(32):4979-4987. doi: 10.1016/j.vaccine.2020.05.074. Epub 2020 Jun 11. Vaccine. 2020. PMID: 32536549
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
Cited by
-
Mycoplasma bovis 5'-nucleotidase is a virulence factor conferring mammary fitness in bovine mastitis.PLoS Pathog. 2024 Nov 12;20(11):e1012628. doi: 10.1371/journal.ppat.1012628. eCollection 2024 Nov. PLoS Pathog. 2024. PMID: 39531484 Free PMC article.
-
Predictive value of immune-related parameters in severe Mycoplasma pneumoniae pneumonia in children.Transl Pediatr. 2024 Sep 30;13(9):1521-1528. doi: 10.21037/tp-24-172. Epub 2024 Sep 13. Transl Pediatr. 2024. PMID: 39399713 Free PMC article.
-
Analysis of cytokine levels, cytological findings, and MP-DNA level in bronchoalveolar lavage fluid of children with Mycoplasma pneumoniae pneumonia.Immun Inflamm Dis. 2023 May;11(5):e849. doi: 10.1002/iid3.849. Immun Inflamm Dis. 2023. PMID: 37249293 Free PMC article.
-
Enhanced insights into the neutrophil-driven immune mechanisms during Mycoplasma pneumoniae infection.Heliyon. 2024 Oct 4;10(21):e38950. doi: 10.1016/j.heliyon.2024.e38950. eCollection 2024 Nov 15. Heliyon. 2024. PMID: 39524902 Free PMC article. Review.
-
COVID-19 Vaccination in Pregnancy: Pilot Study of Plasma MicroRNAs Associated with Inflammatory Cytokines after COVID-19 mRNA Vaccination.Vaccines (Basel). 2024 Jun 14;12(6):658. doi: 10.3390/vaccines12060658. Vaccines (Basel). 2024. PMID: 38932387 Free PMC article.
References
LinkOut - more resources
Full Text Sources

