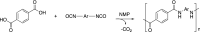A novel treatment and derivatization for quantification of residual aromatic diisocyanates in polyamide resins
- PMID: 35906265
- PMCID: PMC9338256
- DOI: 10.1038/s41598-022-17316-7
A novel treatment and derivatization for quantification of residual aromatic diisocyanates in polyamide resins
Abstract
In the scientific context, the environmental and healthy impact of polymers is more related to the residual monomer content rather than their macromolecular structure, due to the monomer capability to interact with membrane cells. For this a novel method to stabilize and quantify residual monomeric isocyanates in high thermal resistance polyamide resins (PAs) has been developed. This new analytical method resulted in an improvement concerning the quantification of residual aromatic diisocyanates in viscous polymeric matrices by using a simple and cheap technique like HPLC-VWD. Diisocyanate monomers were derivatized with dibutylamine, resulting in stable urea derivatives that were simultaneously analysed and quantified. The method was applied to solvent-based polyamide resins, used as primary electrical insulation, for avoiding additional step of solvent removing before the analysis. The quantification of residual monomers answers to the provisions imposed by European Regulation N. 1907/2006 (REACH) for polymer registration, and the necessity of an early evaluation of the occupational risk associated with the use of diisocyanates, due to their toxicity and high reactivity towards moisture.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Palmer RJ. Polyamides, Plastics. Wiley; 2001.
-
- Barclay MG, Burawoy A, Thomson GH. A new method of nuclear methylation of aromatic amines. J. Chem. Soc. 1944 doi: 10.1039/jr9440000109. - DOI
-
- Ohishi T, Masukawa T, Fujii S, Yokoyama A, Yokozawa T. Synthesis of core cross-linked star polymers consisting of well-defined aromatic polyamide arms. Macromolecules. 2010;43:3206–3214. doi: 10.1021/ma902412t. - DOI
-
- Allard, P. Process for the preparation of aromatic polyamides. US Patent No. 3,642,715 (1975).
-
- Wei Y, Jia X, Jin D, Davis FA. A facile synthesis of polyamides from aromatic diisocyanates and dicarboxylic acid catalyzed by lewis acids. Macromol. Rapid. Commun. 1996;17:897–903. doi: 10.1002/marc.1996.030171208. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources