Metformin treatment is associated with improved outcome in patients with diabetes and advanced heart failure (HFrEF)
- PMID: 35906276
- PMCID: PMC9338272
- DOI: 10.1038/s41598-022-17327-4
Metformin treatment is associated with improved outcome in patients with diabetes and advanced heart failure (HFrEF)
Abstract
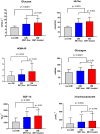
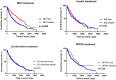
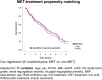
The role of metformin (MET) in the treatment of patients with advanced HFrEF and type 2 diabetes mellitus (DM) is not firmly established. We studied the impact of MET on metabolic profile, quality of life (QoL) and survival in these patients. A total of 847 stable patients with advanced HFrEF (57.4 ± 11.3 years, 67.7% NYHA III/IV, LVEF 23.6 ± 5.8%) underwent clinical and laboratory evaluation and were prospectively followed for a median of 1126 (IQRs 410; 1781) days for occurrence of death, urgent heart transplantation or mechanical circulatory support implantation. A subgroup of 380 patients (44.9%) had DM, 87 of DM patients (22.9%) were treated with MET. Despite worse insulin sensitivity and more severe DM (higher BMI, HbA1c, worse insulin resistance), MET-treated patients exhibited more stable HF marked by lower BNP level (400 vs. 642 ng/l), better LV and RV function, lower mitral and tricuspid regurgitation severity, were using smaller doses of diuretics (all p < 0.05). Further, they had higher eGFR (69.23 vs. 63.34 ml/min/1.73 m2) and better QoL (MLHFQ: 36 vs. 48 points, p = 0.002). Compared to diabetics treated with other glucose-lowering agents, MET-treated patients had better event-free survival even after adjustment for BNP, BMI and eGFR (p = 0.035). Propensity score-matched analysis with 17 covariates yielded 81 pairs of patients and showed a significantly better survival for MET-treated subgroup (p = 0.01). MET treatment in patients with advanced HFrEF and DM is associated with improved outcome by mechanisms beyond the improvement of blood glucose control.
© 2022. The Author(s).
Conflict of interest statement
Josef Kautzner is a member of Advisory Boards for Bayer, Boehringer Ingelheim, Daiichi Sankyo, Biosense Webster, Medtronic and St Jude Medical (Abbott). He has received speaker honoraria from the above-mentioned companies and from Biotronik, Mylan, Pfizer and Pro Med. Petr Jarolim received research support from Abbott Laboratories, Amgen Inc., AstraZeneca LP, Beckman Coulter, Daiichi Sankyo, Inc., GlaxoSmithKline, Merck & Co., Inc., Roche Diagnostics Corporation, Takeda Global Research and Development Center and Waters Technologies Corporation and speaker honoraria from Roche Diagnostics Corporation. All other authors have no competing interest to declare.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

