MicroRNA-193a-5p Rescues Ischemic Cerebral Injury by Restoring N2-Like Neutrophil Subsets
- PMID: 35906328
- PMCID: PMC10299937
- DOI: 10.1007/s12975-022-01071-y
MicroRNA-193a-5p Rescues Ischemic Cerebral Injury by Restoring N2-Like Neutrophil Subsets
Abstract
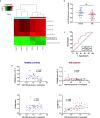
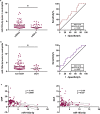
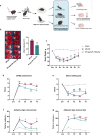
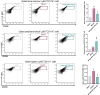
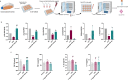
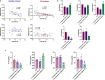
Circulating neutrophils are activated shortly after stroke and in turn affect the fate of ischemic brain tissue, and microRNAs (miRNA) participate in regulating neuroinflammation. We probed the role of neutrophilic miRNA in ischemic stroke. miR-193a-5p was decreased in circulating neutrophils of acute ischemic stroke (AIS) patients and healthy controls. In another set of AIS patients treated with recombinant tissue plasminogen activator, higher neutrophilic miR-193a-5p levels were associated with favorable outcomes at 3 months and non-symptomatic intracerebral hemorrhage. An experimental stroke model and human neutrophil-like HL-60 cells were further transfected with agomiR-193a-5p/antagomiR-193a-5p or ubiquitin-conjugating enzyme V2 (UBE2V2)-siRNA prior to model induction for in vivo and in vitro studies. Results of 2,3,5-triphenyl tetrazolium chloride staining and neurological function evaluations at post-experimental stroke showed that intravenous agomiR-193a-5p transfusion protected against ischemic cerebral injury in the acute stage and promoted neurological recovery in the subacute stage. This protective role was suggested to correlate with neutrophil N2 transformation based on the N2-like neutrophil proportions in the bone marrow, peripheral blood, and spleen of the experimental stroke model and the measurement of neutrophil phenotype-associated molecule levels. Mechanistically, analyses indicated that UBE2V2 might be a target of miR-193a-5p. Cerebral injury and neuroinflammation aggravated by miR-193a-5p inhibition were reversed by UBE2V2 silencing. In conclusion, miR-193a-5p protects against cerebral ischemic injury by restoring neutrophil N2 phenotype-associated neuroinflammation suppression, likely, in part, via UBE2V2 induction.
Keywords: Cerebral ischemic injury; N2-like neutrophil; UBE2V2; miR-193a-5p.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Enlimomab Acute Stroke Trial Investigators. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001;57:1428–1434. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
