What role does the seed coat play during symbiotic seed germination in orchids: an experimental approach with Dendrobium officinale
- PMID: 35906552
- PMCID: PMC9336064
- DOI: 10.1186/s12870-022-03760-0
What role does the seed coat play during symbiotic seed germination in orchids: an experimental approach with Dendrobium officinale
Abstract
Background: Orchids require specific mycorrhizal associations for seed germination. During symbiotic germination, the seed coat is the first point of fungal attachment, and whether the seed coat plays a role in the identification of compatible and incompatible fungi is unclear. Here, we compared the effects of compatible and incompatible fungi on seed germination, protocorm formation, seedling development, and colonization patterns in Dendrobium officinale; additionally, two experimental approaches, seeds pretreated with NaClO to change the permeability of the seed coat and fungi incubated with in vitro-produced protocorms, were used to assess the role of seed coat played during symbiotic seed germination.
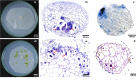
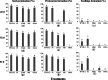
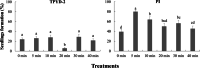
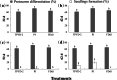
Results: The two compatible fungi, Tulasnella sp. TPYD-2 and Serendipita indica PI could quickly promote D. officinale seed germination to the seedling stage. Sixty-two days after incubation, 67.8 ± 5.23% of seeds developed into seedlings with two leaves in the PI treatment, which was significantly higher than that in the TPYD-2 treatment (37.1 ± 3.55%), and massive pelotons formed inside the basal cells of the protocorm or seedlings in both compatible fungi treatments. In contrast, the incompatible fungus Tulasnella sp. FDd1 did not promote seed germination up to seedlings at 62 days after incubation, and only a few pelotons were occasionally observed inside the protocorms. NaClO seed pretreatment improved seed germination under all three fungal treatments but did not improve seed colonization or promote seedling formation by incompatible fungi. Without the seed coat barrier, the colonization of in vitro-produced protocorms by TPYD-2 and PI was slowed, postponing protocorm development and seedling formation compared to those in intact seeds incubated with the same fungi. Moreover, the incompatible fungus FDd1 was still unable to colonize in vitro-produced protocorms and promote seedling formation.
Conclusions: Compatible fungi could quickly promote seed germination up to the seedling stage accompanied by hyphal colonization of seeds and formation of many pelotons inside cells, while incompatible fungi could not continuously colonize seeds and form enough protocorms to support D. officinale seedling development. The improvement of seed germination by seed pretreatment may result from improving the seed coat hydrophilicity and permeability, but seed pretreatment cannot change the compatibility of a fungus with an orchid. Without a seed coat, the incompatible fungus FDd1 still cannot colonize in vitro-produced protocorms or support seedling development. These results suggest that seed coats are not involved in symbiotic germination in D. officinale.
Keywords: Compatible fungi; Dust seeds; Incompatible fungi; Orchid mycorrhizal fungi; Plant-fungus interactions; Symbiotic seed germination.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Compatible and Incompatible Mycorrhizal Fungi With Seeds of Dendrobium Species: The Colonization Process and Effects of Coculture on Germination and Seedling Development.Front Plant Sci. 2022 Mar 10;13:823794. doi: 10.3389/fpls.2022.823794. eCollection 2022. Front Plant Sci. 2022. PMID: 35360307 Free PMC article.
-
Fungi isolated from host protocorms accelerate symbiotic seed germination in an endangered orchid species (Dendrobium chrysotoxum) from southern China.Mycorrhiza. 2020 Jul;30(4):529-539. doi: 10.1007/s00572-020-00964-w. Epub 2020 Jun 19. Mycorrhiza. 2020. PMID: 32562087
-
Host-specificity of symbiotic mycorrhizal fungi for enhancing seed germination, protocorm formation and seedling development of over-collected medicinal orchid, Dendrobium devonianum.J Microbiol. 2018 Jan;56(1):42-48. doi: 10.1007/s12275-018-7225-1. Epub 2018 Jan 4. J Microbiol. 2018. PMID: 29299845
-
Orchid Reintroduction Based on Seed Germination-Promoting Mycorrhizal Fungi Derived From Protocorms or Seedlings.Front Plant Sci. 2021 Jun 30;12:701152. doi: 10.3389/fpls.2021.701152. eCollection 2021. Front Plant Sci. 2021. PMID: 34276753 Free PMC article. Review.
-
Symbiotic in vitro seed propagation of Dendrobium: fungal and bacterial partners and their influence on plant growth and development.Planta. 2015 Jul;242(1):1-22. doi: 10.1007/s00425-015-2301-9. Epub 2015 May 5. Planta. 2015. PMID: 25940846 Review.
Cited by
-
Plant-Fungi Mutualism, Alternative Splicing, and Defense Responses: Balancing Symbiosis and Immunity.Int J Mol Sci. 2025 May 28;26(11):5197. doi: 10.3390/ijms26115197. Int J Mol Sci. 2025. PMID: 40508007 Free PMC article. Review.
-
Ionome mapping and amino acid metabolome profiling of Phaseolus vulgaris L. seeds imbibed with computationally informed phytoengineered copper sulphide nanoparticles.Discov Nano. 2024 Jan 4;19(1):8. doi: 10.1186/s11671-023-03953-y. Discov Nano. 2024. PMID: 38175418 Free PMC article.
-
Gibberellic Acid Inhibits Dendrobium nobile-Piriformospora Symbiosis by Regulating the Expression of Cell Wall Metabolism Genes.Biomolecules. 2023 Nov 14;13(11):1649. doi: 10.3390/biom13111649. Biomolecules. 2023. PMID: 38002331 Free PMC article.
-
The orchid seed coat: a developmental and functional perspective.Bot Stud. 2023 Sep 27;64(1):27. doi: 10.1186/s40529-023-00400-0. Bot Stud. 2023. PMID: 37755558 Free PMC article. Review.
-
A novel method to produce massive seedlings via symbiotic seed germination in orchids.Front Plant Sci. 2023 Mar 9;14:1114105. doi: 10.3389/fpls.2023.1114105. eCollection 2023. Front Plant Sci. 2023. PMID: 36968353 Free PMC article.
References
-
- Smith SE, Read DJ. Mycorrhizal Symbiosis. Cambridge, UK: Academic Press; 2008.
-
- Dearnaley J, Perotto S, Selosse M-A. Structure and development of orchid mycorrhizas. In: Martin F, editor. Molecular mycorrhizal symbiosis. Hoboken, NJ: John Wiley and Sons; 2016. pp. 63–86.
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

