Natural history of Type 1 spinal muscular atrophy: a retrospective, global, multicenter study
- PMID: 35906608
- PMCID: PMC9336055
- DOI: 10.1186/s13023-022-02455-x
Natural history of Type 1 spinal muscular atrophy: a retrospective, global, multicenter study
Abstract
Background: ANCHOVY was a global, multicenter, chart-review study that aimed to describe the natural history of Type 1 spinal muscular atrophy (SMA) from a broad geographical area and provide further contextualization of results from the FIREFISH (NCT02913482) interventional study of risdiplam treatment in Type 1 SMA.
Methods: Data were extracted from medical records of patients with first symptoms attributable to Type 1 SMA between 28 days and 3 months of age, genetic confirmation of SMA, and confirmed survival of motor neuron 2 copy number of two or unknown. The study period started on 1 January 2008 for all sites; study end dates were site-specific due to local treatment availabilities. Primary endpoints were time to death and/or permanent ventilation and proportion of patients achieving motor milestones. Secondary endpoints included time to initiation of respiratory and feeding support.
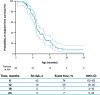
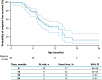
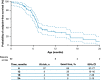
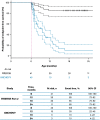
Results: Data for 60 patients from nine countries across Asia, Europe and North and South America were analyzed. The median age (interquartile range [IQR]) for reaching death or permanent ventilation was ~ 7.3 (5.9-10.5) months. The median age (IQR) at permanent ventilation was ~ 12.7 (6.9-16.4) months and at death was ~ 41.2 (7.3-not applicable) months. No patients were able to sit without support or achieved any level of crawling, standing or walking.
Interpretation: Findings from ANCHOVY were consistent with published natural history data on Type 1 SMA demonstrating the disease's devastating course, which markedly differed from risdiplam-treated infants (FIREFISH Part 2). The results provide meaningful additions to the literature, including a broader geographical representation.
Keywords: ANCHOVY; FIREFISH; SMA natural history; Spinal muscular atrophy; Type 1 SMA.
© 2022. The Author(s).
Conflict of interest statement
CC is a site principal investigator for Biogen and F. Hoffmann-La Roche Ltd clinical trials, and has received advisory fees from Novartis TG. VD and GPC have no conflicts of interest. RM has received fees from Biogen, F. Hoffmann-La Roche Ltd and Novartis Gene Therapies. MMB is a site principal investigator for Biogen and F. Hoffmann-La Roche Ltd clinical trials, and has received honoraria for advisory boards and speaker's fees from Biogen, F. Hoffmann-La Roche Ltd, and Novartis. KS is a site principal investigator for Biogen and Novartis Gene Therapies clinical trials, has received honoraria for advisory boards from Biogen, Novartis, and Roche/Chugai and speaker’s fees from Biogen and Novartis. IG and JH are employees of F. Hoffmann-La Roche Ltd. AD, MEK, KG and RSS are employees of, and hold shares in, F. Hoffmann-La Roche Ltd. BTD has received grants from Biogen, CureSMA, F. Hoffmann-La Roche Ltd, Fibrogen, Ionis Pharmaceuticals, U.S. National Institutes of Health/National Institute of Neurological Disorders and Stroke, PTC Therapeutics, Sarepta Pharmaceuticals, Slaney Family Fund for SMA, Spinal Muscular Atrophy Foundation, Summit and Working on Walking Fund; and is a board member for Amicus Inc., AveXis, Biogen, F. Hoffmann-La Roche Ltd grants, Genentech, Sarepta Pharmaceuticals and Vertex.
Figures
References
-
- Darras B, Monani U, De Vivo D. Genetic disorders affecting the motor neuron: spinal muscular atrophy. In: Swaiman K, Ashwal S, Ferriero D, Schor N, Finkel R, Gropman A, editors. Swaiman’s pediatric neurology: principles and practice. 6. Elsevier; 2017. pp. 1057–1064.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

