Dose escalation based on 18F-FDG PET/CT response in definitive chemoradiotherapy of locally advanced esophageal squamous cell carcinoma: a phase III, open-label, randomized, controlled trial (ESO-Shanghai 12)
- PMID: 35906623
- PMCID: PMC9338557
- DOI: 10.1186/s13014-022-02099-y
Dose escalation based on 18F-FDG PET/CT response in definitive chemoradiotherapy of locally advanced esophageal squamous cell carcinoma: a phase III, open-label, randomized, controlled trial (ESO-Shanghai 12)
Abstract
Introduction: Definitive chemoradiotherapy has established the standard non-surgical treatment for locally advanced esophageal cancer. The standard dose of 50-50.4 Gy has been established decades ago and been confirmed in modern trials. The theorical advantage of better local control and technical advances for less toxicity have encouraged clinicians for dose escalation investigation. 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) have the potential to tailor therapy for esophageal patients not showing response to CRT and pioneers the PET-based dose escalation.
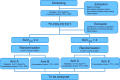
Methods and analysis: The ESO-Shanghai 12 trial is a prospective multicenter randomized phase 3 study in which patients are randomized to either 61.2 Gy or 50.4 Gy of radiation dose by PET response. Both groups undergo concurrent chemoradiotherapy with paclitaxel/cisplatin regimen for 2 cycles followed by consolidation chemotherapy for 2 cycles. Patients with histologically confirmed ESCC [T1N1-3M0, T2-4NxM0, TxNxM1 (Supraclavicular lymph node metastasis only), (AJCC Cancer Staging Manual, 8th Edition)] and without any prior treatment of chemotherapy, radiotherapy or surgery against esophageal cancer will be eligible. The primary endpoints included overall survival in PET/CT non-responders (SUVmax > 4.0) and overall survival in total population. Patients will be stratified by standardized uptake volume, gross tumor volume and tumor location. The enrollment could be ended, when the number of PET/CT non-responder reached 132 and the total population reached 646 for randomization.
Ethics and dissemination: This trial has been approved by the Fudan University Shanghai Cancer Center Institutional Review Board. Trial results will be disseminated via peer reviewed scientific journals and conference presentations. Trial registration The trial was initiated in 2018 and is currently recruiting patients. Trial registration number NCT03790553.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no financial or non-financial competing interests.
Figures

References
-
- Global Burden of Disease 2019 Cancer Collaboration. Kocarnik JM, Compton K, et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer Groups From 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol. 2021;30:e216987. - PMC - PubMed
-
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. - DOI - PMC - PubMed
-
- GBD 2017 Oesophageal Cancer Collaborators The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2020;5(6):582–597. doi: 10.1016/S2468-1253(20)30007-8. - DOI - PMC - PubMed
-
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85–01) Radiat Ther Oncol Gr JAMA. 1999;281(17):1623–1627. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

