Development of a core outcome set and outcome definitions for studies on uterus-sparing treatments of adenomyosis (COSAR): an international multistakeholder-modified Delphi consensus study
- PMID: 35906919
- PMCID: PMC9433836
- DOI: 10.1093/humrep/deac166
Development of a core outcome set and outcome definitions for studies on uterus-sparing treatments of adenomyosis (COSAR): an international multistakeholder-modified Delphi consensus study
Abstract
Study question: What outcomes should be reported in all studies investigating uterus-sparing interventions for treating uterine adenomyosis?
Summary answer: We identified 24 specific and 26 generic core outcomes in nine domains.
What is known already: Research reporting adenomyosis treatment is not patient-centred and shows wide variation in outcome selection, definition, reporting and measurement of quality.
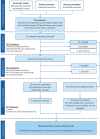
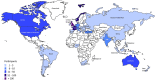
Study design, size, duration: An international consensus development process was performed between March and December 2021. Participants in round one were 150 healthcare professionals, 17 researchers and 334 individuals or partners with lived experience of adenomyosis from 48 high-, middle- and low-income countries. There were 291 participants in the second round.
Participants/materials, setting, methods: Stakeholders included active researchers in the field, healthcare professionals involved in diagnosis and treatment, and people and their partners with lived experience of adenomyosis. The core component of the process was a 2-step modified Delphi electronic survey. The Steering Committee analysed the results and created the final core outcome set (COS) in a semi-structured meeting.
Main results and the role of chance: A total of 241 outcomes was identified and distilled into a 'long list' of 71 potential outcomes. The final COS comprises 24 specific and 26 generic core outcomes across nine domains, including pain, uterine bleeding, reproductive outcomes, haematology, urinary system, life impact, delivery of care, adverse events and reporting items, all with definitions provided by the Steering Committee. Nineteen of these outcomes will apply only to certain study types. Although not included in the COS, the Steering Committee recommended that three health economic outcomes should be recorded.
Limitations, reasons for caution: Patients from continents other than Europe were under-represented in this survey. A lack of translation of the survey might have limited the active participation of people in non-English speaking countries. Only 58% of participants returned to round two, but analysis did not indicate attrition bias. There is a significant lack of scientific evidence regarding which symptoms are caused by adenomyosis and when they are related to other co-existent disorders such as endometriosis. As future research provides more clarity, the appropriate review and revision of the COS will be necessary.
Wider implications of the findings: Implementing this COS in future studies on the treatment of adenomyosis will improve the quality of reporting and aid evidence synthesis.
Study funding/competing interest(s): No specific funding was received for this work. T.T. received a grant (grant number 2020083) from the South Eastern Norwegian Health Authority during the course of this work. T.T. receives personal fees from General Electrics and Medtronic for lectures on ultrasound. E.R.L. is the chairman of the Norwegian Endometriosis Association. M.G.M. is a consultant for Abbvie Inc and Myovant, receives research funding from AbbVie and is Chair of the Women's Health Research Collaborative. S.-W.G. is a board member of the Asian Society of Endometriosis and Adenomyosis, on the scientific advisory board of the endometriosis foundation of America, previous congress chair for the World Endometriosis Society, for none of which he received personal fees. E.S. received outside of this work grants for two multicentre trials on endometriosis from the National Institute for Health Research UK, the Rosetrees Trust, and the Barts and the London Charity, he is a member of the Medicines and Healthcare Products Regulatory Agency (MHRA), Medicines for Women's Health Expert Advisory Group, he is an ambassador for the World Endometriosis Society, and he received personal fees for lectures from Hologic, Olympus, Medtronic, Johnson & Johnson, Intuitive and Karl Storz. M.H. is member of the British Society for Gynaecological Endoscopy subcommittee. No other conflict of interest was declared.
Trial registration number: N/A.
Keywords: Delphi; adenomyosis; core outcome set; outcomes; patient centredness; quality of care; quality of life; reporting; stakeholder.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures
Similar articles
-
ESHRE guideline: endometriosis.Hum Reprod Open. 2022 Feb 26;2022(2):hoac009. doi: 10.1093/hropen/hoac009. eCollection 2022. Hum Reprod Open. 2022. PMID: 35350465 Free PMC article.
-
A core outcome set for future male infertility research: development of an international consensus.Hum Reprod. 2025 May 1;40(5):865-875. doi: 10.1093/humrep/deaf039. Hum Reprod. 2025. PMID: 40233940 Free PMC article.
-
Evidence-based guideline: premature ovarian insufficiency.Hum Reprod Open. 2024 Dec 9;2024(4):hoae065. doi: 10.1093/hropen/hoae065. eCollection 2024. Hum Reprod Open. 2024. PMID: 39660328 Free PMC article.
-
Top 10 priorities for future infertility research: an international consensus development study.Fertil Steril. 2021 Jan;115(1):180-190. doi: 10.1016/j.fertnstert.2020.11.014. Epub 2020 Nov 30. Fertil Steril. 2021. PMID: 33272617
-
A systematic review of outcome reporting and outcome measures in studies investigating uterine-sparing treatment for adenomyosis.Hum Reprod Open. 2021 Aug 7;2021(3):hoab030. doi: 10.1093/hropen/hoab030. eCollection 2021. Hum Reprod Open. 2021. PMID: 34466664 Free PMC article. Review.
Cited by
-
Knowledge, attitudes, and practices among Chinese reproductive-age women toward uterine adenomyosis.Front Med (Lausanne). 2024 Apr 8;11:1361671. doi: 10.3389/fmed.2024.1361671. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38651069 Free PMC article.
-
Radiofrequency Ablation for Adenomyosis.J Clin Med. 2023 Apr 23;12(9):3069. doi: 10.3390/jcm12093069. J Clin Med. 2023. PMID: 37176514 Free PMC article. Review.
-
Quality of life 1 year after uterine artery embolization vs hysterectomy for symptomatic adenomyosis (QUESTA study).Acta Obstet Gynecol Scand. 2025 Aug;104(8):1558-1574. doi: 10.1111/aogs.15165. Epub 2025 May 26. Acta Obstet Gynecol Scand. 2025. PMID: 40420328 Free PMC article. Clinical Trial.
-
Treatment options for women with heavy menstrual bleeding: a protocol for comprehensive systematic review, network meta-analyses and health economic assessment.BMJ Open. 2025 Apr 22;15(4):e085292. doi: 10.1136/bmjopen-2024-085292. BMJ Open. 2025. PMID: 40262956 Free PMC article.
-
In Search of an Imaging Classification of Adenomyosis: A Role for Elastography?J Clin Med. 2022 Dec 30;12(1):287. doi: 10.3390/jcm12010287. J Clin Med. 2022. PMID: 36615089 Free PMC article. Review.
References
-
- Beckstead JW. On measurements and their quality. Paper 4: verbal anchors and the number of response options in rating scales. Int J Nurs Stud 2014;51:807–814. - PubMed
-
- Bourdon M, Santulli P, Oliveira J, Marcellin L, Maignien C, Melka L, Bordonne C, Millisher AE, Plu-Bureau G, Cormier J. et al. Focal adenomyosis is associated with primary infertility. Fertil Steril 2020;114:1271–1277. - PubMed
-
- Bruun MR, Arendt LH, Forman A, Ramlau-Hansen CH.. Endometriosis and adenomyosis are associated with increased risk of preterm delivery and a small-for-gestational-age child: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2018;97:1073–1090. - PubMed
-
- Centers for Disease Control and Prevention. Measuring Healthy Days. Atlanta, GA: CDC, 2000. https://www.cdc.gov/hrqol/pdfs/mhd.pdf (1 May 2022, date last accessed).