Aging the brain: multi-region methylation principal component based clock in the context of Alzheimer's disease
- PMID: 35907208
- PMCID: PMC9365556
- DOI: 10.18632/aging.204196
Aging the brain: multi-region methylation principal component based clock in the context of Alzheimer's disease
Abstract
Alzheimer's disease (AD) risk increases exponentially with age and is associated with multiple molecular hallmarks of aging, one of which is epigenetic alterations. Epigenetic age predictors based on 5' cytosine methylation (DNAm), or epigenetic clocks, have previously suggested that epigenetic age acceleration may occur in AD brain tissue. Epigenetic clocks are promising tools for the quantification of biological aging, yet we hypothesize that investigation of brain aging in AD will be assisted by the development of brain-specific epigenetic clocks. Therefore, we generated a novel age predictor termed PCBrainAge that was trained solely in cortical samples. This predictor utilizes a combination of principal components analysis and regularized regression, which reduces technical noise and greatly improves test-retest reliability. To characterize the scope of PCBrainAge's utility, we generated DNAm data from multiple brain regions in a sample from the Religious Orders Study and Rush Memory and Aging Project. PCBrainAge captures meaningful heterogeneity of aging: Its acceleration demonstrates stronger associations with clinical AD dementia, pathologic AD, and APOE ε4 carrier status compared to extant epigenetic age predictors. It further does so across multiple cortical and subcortical regions. Overall, PCBrainAge's increased reliability and specificity makes it a particularly promising tool for investigating heterogeneity in brain aging, as well as epigenetic alterations underlying AD risk and resilience.
Keywords: Alzheimer's disease; age acceleration; brain; epigenetic clocks; unsupervised machine learning.
Conflict of interest statement
Figures



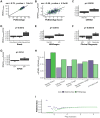
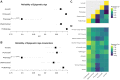
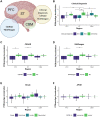

References
-
- Kerchner GA, Wyss-Coray T. The Role of Aging in Alzheimer’s Disease. In: Sierra F, Kohanski R. (eds) Advances in Geroscience. Springer: Cham. 2016; 197–227. 10.1007/978-3-319-23246-1_7 - DOI
Publication types
MeSH terms
Grants and funding
- P30 AG072975/AG/NIA NIH HHS/United States
- F31 AG074627/AG/NIA NIH HHS/United States
- U01 AG061356/AG/NIA NIH HHS/United States
- P30 AG066508/AG/NIA NIH HHS/United States
- R01 AG057911/AG/NIA NIH HHS/United States
- R01 AG057912/AG/NIA NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
- R01 AG061798/AG/NIA NIH HHS/United States
- RF1 AG036042/AG/NIA NIH HHS/United States
- U01 AG046152/AG/NIA NIH HHS/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- T32 MH019961/MH/NIMH NIH HHS/United States
- R01 AG036042/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

