CD169+ subcapsular sinus macrophage-derived microvesicles are associated with light zone follicular dendritic cells
- PMID: 35907260
- PMCID: PMC9804338
- DOI: 10.1002/eji.202249879
CD169+ subcapsular sinus macrophage-derived microvesicles are associated with light zone follicular dendritic cells
Abstract
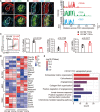
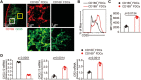
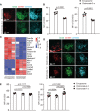
Follicular dendritic cells (FDCs) are a specialized type of stromal cells that exclusively reside in B-cell follicles. When inflammation occurs, the FDC network is reorganized to support germinal center (GC) polarization into the light zone (LZ) and dark zone (DZ). Despite the indispensable role of FDCs in supporting humoral responses, the FDC regulatory requirements remain incompletely defined. In this study, we unexpectedly observed an accumulation of CD169+ subcapsular sinus macrophage (SSM)-derived microvesicles (MVs) in the B-cell zone, which were tightly associated with the FDC network. Interestingly, a selective deposition of CD169+ MVs was detected in both GC LZ FDCs in secondary follicles and on predetermined LZ FDCs in primary follicles. The ablation of CD169+ MVs, resulting from SSM depletion, resulted in significantly decreased expression of LZ-related genes in FDCs. In addition, we found that CD169+ MVs could colocalize with fluorescently tagged antigen-containing immune complexes (ICs), supporting a possible role of CD169+ MVs in transporting antigens to the FDC network. Thus, our data reveal intimate crosstalk between FDCs and SSMs located outside B-cell follicles via SSM-released MVs, providing a novel perspective on the mechanisms underlying the regulation of FDC maturation and polarization.
Keywords: CD169+ MVs; FDCs; ICs; LZ; SSMs.
© 2022 The Authors. European Journal of Immunology published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no commercial or financial conflict of interests.
Figures



Similar articles
-
Long-term retention of antigens in germinal centers is controlled by the spatial organization of the follicular dendritic cell network.Nat Immunol. 2023 Aug;24(8):1281-1294. doi: 10.1038/s41590-023-01559-1. Epub 2023 Jul 13. Nat Immunol. 2023. PMID: 37443283 Free PMC article.
-
A Novel Image Analysis Approach Reveals a Role for Complement Receptors 1 and 2 in Follicular Dendritic Cell Organization in Germinal Centers.Front Immunol. 2021 Apr 12;12:655753. doi: 10.3389/fimmu.2021.655753. eCollection 2021. Front Immunol. 2021. PMID: 33912182 Free PMC article.
-
IL-6 produced by immune complex-activated follicular dendritic cells promotes germinal center reactions, IgG responses and somatic hypermutation.Int Immunol. 2009 Jun;21(6):745-56. doi: 10.1093/intimm/dxp041. Epub 2009 May 21. Int Immunol. 2009. PMID: 19461124 Free PMC article.
-
Follicular dendritic cells and germinal centers.Int Rev Cytol. 1996;166:139-79. doi: 10.1016/s0074-7696(08)62508-5. Int Rev Cytol. 1996. PMID: 8881775 Review.
-
Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function.Semin Immunol. 2008 Feb;20(1):14-25. doi: 10.1016/j.smim.2007.12.001. Epub 2008 Feb 7. Semin Immunol. 2008. PMID: 18261920 Free PMC article. Review.
Cited by
-
Holistic View on the Structure of Immune Response: Petri Net Model.Biomedicines. 2023 Feb 4;11(2):452. doi: 10.3390/biomedicines11020452. Biomedicines. 2023. PMID: 36830988 Free PMC article.
-
CD169+ Macrophages in Primary Breast Tumors Associate with Tertiary Lymphoid Structures, Tregs and a Worse Prognosis for Patients with Advanced Breast Cancer.Cancers (Basel). 2023 Feb 16;15(4):1262. doi: 10.3390/cancers15041262. Cancers (Basel). 2023. PMID: 36831605 Free PMC article.
-
Difference between sentinel and non-sentinel lymph nodes in the distribution of dendritic cells and macrophages: An immunohistochemical and morphometric study using gastric regional nodes obtained in sentinel node navigation surgery for early gastric cancer.J Anat. 2025 Feb;246(2):272-287. doi: 10.1111/joa.14147. Epub 2024 Oct 5. J Anat. 2025. PMID: 39367691 Free PMC article.
-
Altered immune signatures in breast cancer lymph nodes with metastases revealed by spatial proteome analyses.J Transl Med. 2025 Apr 10;23(1):422. doi: 10.1186/s12967-025-06415-4. J Transl Med. 2025. PMID: 40211433 Free PMC article.
References
-
- Van den Broeck, W. , Derore, A. and Simoens, P. , Anatomy and nomenclature of murine lymph nodes: Descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J Immunol Methods 2006. 312: 12‐19. - PubMed
-
- Link, A. , Vogt, T. K. , Favre, S. , Britschgi, M. R. , Acha‐Orbea, H. , Hinz, B. , Cyster, J. G. and Luther, S. A. , Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol 2007. 8: 1255‐1265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

