Safety and immunogenicity of a simian-adenovirus-vectored rabies vaccine: an open-label, non-randomised, dose-escalation, first-in-human, single-centre, phase 1 clinical trial
- PMID: 35907430
- PMCID: PMC7614839
- DOI: 10.1016/S2666-5247(22)00126-4
Safety and immunogenicity of a simian-adenovirus-vectored rabies vaccine: an open-label, non-randomised, dose-escalation, first-in-human, single-centre, phase 1 clinical trial
Abstract
Background: Rabies kills around 60 000 people each year. ChAdOx2 RabG, a simian adenovirus-vectored rabies vaccine candidate, might have potential to provide low-cost single-dose pre-exposure rabies prophylaxis. This first-in-human study aimed to evaluate its safety and immunogenicity in healthy adults.
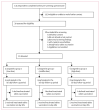
Methods: We did a single-centre phase 1 study of ChAdOx2 RabG, administered as a single intramuscular dose, with non-randomised open-label dose escalation at the Centre for Clinical Vaccinology and Tropical Medicine, Oxford, UK. Healthy adults were sequentially allocated to groups receiving low (5 × 109 viral particles), middle (2·5 × 1010 viral particles), and high doses (5 x 1010 viral particles) of ChAdOx2 RabG and were followed up to day 56 after vaccination. The primary objective was to assess safety. The secondary objective was to assess immunogenicity with the internationally standardised rabies virus neutralising antibody assay. In an optional follow-up phase 1 year after enrolment, we measured antibody maintenance then administered a licensed rabies vaccine (to simulate post-exposure prophylaxis) and measured recall responses. The trial is registered with ClinicalTrials.gov, NCT04162600, and is now closed to new participants.
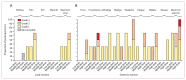
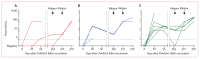
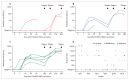
Findings: Between Jan 2 and Oct 28, 2020, 12 adults received low (n=3), middle (n=3), and high doses (n=6) of ChAdOx2 RabG. Participants reported predominantly mild-to-moderate reactogenicity. There were no serious adverse events. Virus neutralising antibody concentrations exceeded the recognised correlate of protection (0·5 IU/mL) in three middle-dose recipients and six high-dose recipients within 56 days of vaccination (median 18·0 IU/mL). The median peak virus neutralising antibody concentrations within 56 days were 0·7 IU/mL (range 0·0-54·0 IU/mL) for the low-dose group, 18·0 IU/mL (0·7-18·0 IU/mL) for the middle-dose group, and 18·0 IU/mL (6·0-486·0 IU/mL) for the high-dose group. Nine participants returned for the additional follow-up after 1 year. Of these nine participants, virus neutralising antibody titres of more than 0·5 IU/mL were maintained in six of seven who had received middle-dose or high-dose ChAdOx2 RabG. Within 7 days of administration of the first dose of a licensed rabies vaccine, nine participants had virus neutralising antibody titres of more than 0·5 IU/mL.
Interpretation: In this study, ChAdOx2 RabG showed an acceptable safety and tolerability profile and encouraging immunogenicity, supporting further clinical evaluation.
Funding: UK Medical Research Council and Engineering and Physical Sciences Research Council.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests AJR might receive royalties arising from the University of Oxford— AstraZeneca COVID-19 vaccine, which also uses the chimpanzee adenovirus technology platform. SF has received payment from Merck for a presentation at the ISPE Virtual Annual Conference, and is a contributor to intellectual property assigned to Oxford University Innovation relating to the ChAdOx1 nCoV-19 vaccine and might receive a proportion of proceeds from out-licensing of the property. CG has received a personal honorarium from the Duke Human Vaccine Institute ISAB and is director of Vaxxers. HCJE reports funding from the Wellcome Trust; grants from the US Department of Defense, Virion Therapeutics, Corona Discovery Fund, and Commonwealth of Pennsylvania, USA; research funding from Virion Therapeutics; consulting fees from Takeda, Biogen, RegenXBio; support for attending meetings or travel from Society for Immunotherapy of Cancer; support from Virion Therapeutics for travel to the Genetic Vaccine Development for Infectious Diseases Summit, Boston, MA, USA; has patents filed (US Patent 11291716 adenoviral vectors encoding hepatitis B viral antigens fused to herpes virus glycoprotein D and methods of using the same; and US Patent 11207402 constructs for enhancing immune responses); and has stock options in Ring Therapeutics. ADD reports grant funding from the Medical Research Council, Engineering and Physical Sciences Research Council, and the Wellcome Trust; might receive income arising from licensing of intellectual property related to ChAdOx2 RabG or other adenovirus-vectored vaccines; has received consultancy fees from AstraZeneca, relating to another adenovirus-vectored vaccine; and is a named inventor on patent applications relating to chimpanzee adenovirus platform technology. All other authors declare no competing interests.
Figures
References
-
- WHO. WHO expert consultation on rabies: third report. World Health Organization; Geneva: 2018.
-
- WHO. Rabies vaccines: WHO position paper, April 2018. WHO Weekly Epidemiological Record. 2018;93:201–20.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

