Subcutaneous administration, higher age and lower renal function are associated with erythrocyte methotrexate accumulation in Crohn's disease: a cross-sectional study
- PMID: 35907797
- PMCID: PMC9338675
- DOI: 10.1186/s12876-022-02439-y
Subcutaneous administration, higher age and lower renal function are associated with erythrocyte methotrexate accumulation in Crohn's disease: a cross-sectional study
Abstract
Background: Methotrexate is an immunomodulatory drug for patients with Crohn's disease. Erythrocyte MTX-polyglutamates (MTX-PG1-5) may be used for therapeutic drug monitoring (TDM) as MTX-PG is thought to mediate MTX's efficacy. Information on determinants of the concentration of MTX-PG in patients with Crohn's disease is lacking. We aim to identify clinical and biochemical determinants of the erythrocyte MTX-PG1-5 and MTX-PGtotal concentration in patients with Crohn's disease.
Methods: Adults with Crohn's disease on methotrexate treatment who visited the outpatient clinic of Amsterdam UMC were included. Erythrocyte MTX-PGs were measured by tandem mass spectrometry.
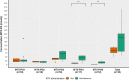
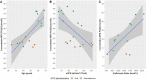
Results: Nineteen patients were included, with a median duration of MTX use of 77 months (range 7-202). Twelve patients received MTX monotherapy, whereas 7 patients were on concomitant TNF-α inhibitors. The mean dose of MTX was 15.5 mg (SD ± 2.8) and 12 (63%) patients used subcutaneous MTX. MTX-PG1-5 were successfully measured in 18 patients, showing substantial variability in concentrations of MTX-PGtotal and individual species. The median MTX-PGtotal was 117.1 nmol/L (range 46.4-258.7) with preferential accumulation of MTX-PG3 (43.1 nmol/L, range 15.3-96.1). Patients on subcutaneous compared to oral MTX had higher median MTX-PG(4,5) levels (55 versus 9 nmol/L, p = 0.01). Higher age (β = 0.71) and lower estimated glomerular filtration rate (β = - 0.52) were associated with a significantly higher MTX-PGtotal concentration (R2 = 0.60, p = 0.001).
Conclusion: MTX-PG concentrations display a considerable inter-individual variability. Higher MTX-PG accumulation is associated with subcutaneous administration, higher age, and lower renal function in Crohn's disease patients.
Keywords: Crohn’s disease; Methotrexate; Pharmacokinetics; Therapeutic drug monitoring.
© 2022. The Author(s).
Conflict of interest statement
The following authors are members of the funding body ICC
Figures
References
-
- Feagan BG, Fedorak RN, Jan Irvine E, Wild G, Sutherland AL, Steinhart H, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s study group investigators. New Engl J Med. 2000;342(22):1627–1632. doi: 10.1056/NEJM200006013422202. - DOI - PubMed
-
- Qiu Y, Mao R, Chen B, et al. Effects of combination therapy with immunomodulators on trough levels and antibodies against tumor necrosis factor antagonists in patients with inflammatory bowel disease: a meta-analysis. Clin Gastroenterol Hepatol. 2017;15(9):1359–72. doi: 10.1016/j.cgh.2017.02.005. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

