Bone morphogenetic protein 4 rescues the bone regenerative potential of old muscle-derived stem cells via regulation of cell cycle inhibitors
- PMID: 35907860
- PMCID: PMC9338549
- DOI: 10.1186/s13287-022-03047-z
Bone morphogenetic protein 4 rescues the bone regenerative potential of old muscle-derived stem cells via regulation of cell cycle inhibitors
Abstract
Background: Bone morphogenetic protein 4 (BMP4) promotes the osteogenic differentiation and the bone regenerative potential of muscle-derived stem cells (MDSCs). BMP4 also promotes the self-renewal of both embryonic and somatic stem cells; however, BMP4 signaling activity significantly decreases with age. Cyclin-dependent kinase inhibitors P16INK4A (P16) and P18INK4C (P18) induce early G1-phase cell cycle blockade by targeting cyclin-dependent kinase 4/6. It is still unclear if BMP4 affects the bone regenerative potential of old MDSCs through regulation of P16 and P18 expression.
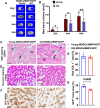
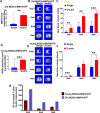
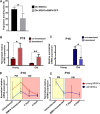
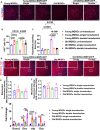
Methods: Young and old MDSCs were isolated from 3 week (young) and 2-year-old (old) mice. In vitro cell proliferation and multipotent differentiation were performed for young and old MDSCs both before and after BMP4/GFP transduction. Cell cycle genes were analyzed using Q-PCR. The bone regenerative potential of young and old MDSCs transduced with BMP4/GFP were compared using Micro-CT and histological analysis. The bone regenerative potential of young and old MDSCs was also compared between single and double transduction (higher BMP4 levels expression). The cell proliferation, mitochondrial function and osteogenic differentiation was also compared in vitro between cells that have been transduced with BMP4GFP (single and double transduction). The correlation of bone regeneration capacity of young and old MDSCs with P16 and P18 expression was further evaluated at 10 days after cell transplantation using histology and western blot analysis.
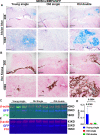
Results: Old murine MDSCs (MDSCs) exhibit reduced proliferation and multi-lineage differentiation potential with or without BMP4 stimulation, when compared to young murine MDSCs. Old MDSCs express significantly higher P16 and lower P18, with more cells in the G0/1 phase and fewer cells in the G2/M phase, compared to young MDSCs. Old MDSCs retrovirally transduced to express BMP4 regenerated less bone in a critical size skull defect in CD-1 nude mice when compared to young retrovirally transduced MDSCs expressing similar BMP4 levels and contribute less to the new regenerated new bone. Importantly, both young and old MDSCs can regenerate more bone when BMP4 expression levels are increased by double-transduction with the retroviral-BMP4/GFP. However, the bone regeneration enhancement with elevated BMP4 was more profound in old MDSCs (400% at 2 weeks) compared to young MDSCs (200%). Accordingly, P18 is upregulated while P16 is downregulated after BMP4 transduction. Double transduction did not further increase cell proliferation nor mitochondrial function but did significantly increase Osx expression in both young and old MDSCs. Old MDSCs had even significant higher Osx levels as compared to young MDSCs following double transduction, while a similar Alp expression was observed between young and old MDSCs after double transduction. In addition, at 10 days after cell transplantation, old MDSCs having undergone double transduction regenerated bone more rapidly as showed by Alcian blue and Von Kossa staining. Western blot assays demonstrated that old MDSCs after retro-BMP4/GFP double transduction have significantly lower P18 expression levels when compared to young BMP4-transduced MDSCs. In addition, P18 expression was slightly increased in old MDSCs after double transduction when compared to single transduction. P16 expression was not detectable for both young and two old BMP4/GFP transduced MDSCs groups.
Conclusions: In summary, BMP4 can offset the adverse effect of aging on the osteogenic differentiation and the bone regenerative potential of old MDSCs via up-regulation of P18 and down-regulation P16 expression.
Keywords: Aging; Bone morphogenetic protein 4; Bone regeneration; Muscle-derived stem cells; P16; P18.
© 2022. The Author(s).
Conflict of interest statement
J.H received Royalties from Cooke Myocytes Inc. All authors declare no competing interest.
Figures




Similar articles
-
BMP2 is superior to BMP4 for promoting human muscle-derived stem cell-mediated bone regeneration in a critical-sized calvarial defect model.Cell Transplant. 2013;22(12):2393-408. doi: 10.3727/096368912X658854. Epub 2012 Nov 1. Cell Transplant. 2013. PMID: 23244588 Free PMC article.
-
Ex vivo gene therapy-induced endochondral bone formation: comparison of muscle-derived stem cells and different subpopulations of primary muscle-derived cells.Bone. 2004 Jun;34(6):982-92. doi: 10.1016/j.bone.2004.01.028. Bone. 2004. PMID: 15193544
-
Osteogenic potential of postnatal skeletal muscle-derived stem cells is influenced by donor sex.J Bone Miner Res. 2007 Oct;22(10):1592-602. doi: 10.1359/jbmr.070702. J Bone Miner Res. 2007. PMID: 17605633
-
Role and Mechanism of BMP4 in Regenerative Medicine and Tissue Engineering.Ann Biomed Eng. 2023 Jul;51(7):1374-1389. doi: 10.1007/s10439-023-03173-6. Epub 2023 Apr 4. Ann Biomed Eng. 2023. PMID: 37014581 Review.
-
The role of BMP4 in adipose-derived stem cell differentiation: A minireview.Front Cell Dev Biol. 2022 Oct 21;10:1045103. doi: 10.3389/fcell.2022.1045103. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36340030 Free PMC article. Review.
Cited by
-
Lamc1 promotes osteogenic differentiation and inhibits adipogenic differentiation of bone marrow-derived mesenchymal stem cells.Sci Rep. 2024 Aug 23;14(1):19592. doi: 10.1038/s41598-024-69629-4. Sci Rep. 2024. PMID: 39179716 Free PMC article.
-
Sex hormone-binding globulin promotes the osteogenic differentiation potential of equine adipose-derived stromal cells by activating the BMP signaling pathway.Front Endocrinol (Lausanne). 2024 Oct 17;15:1424873. doi: 10.3389/fendo.2024.1424873. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39483986 Free PMC article.
-
Trends in bioactivity: inducing and detecting mineralization of regenerative polymeric scaffolds.J Mater Chem B. 2024 Mar 13;12(11):2720-2736. doi: 10.1039/d3tb02674d. J Mater Chem B. 2024. PMID: 38410921 Free PMC article. Review.
-
Bone morphogenetic protein-4 induced matrix turnover and osteogenic differentiation-related molecules of stem cells from apical papilla and its associated ALK/Smad signaling.J Dent Sci. 2025 Jan;20(1):646-659. doi: 10.1016/j.jds.2024.11.002. Epub 2024 Nov 17. J Dent Sci. 2025. PMID: 39873078 Free PMC article.
-
Distribution of Immunomodulation, Protection and Regeneration Factors in Cleft-Affected Bone and Cartilage.Diagnostics (Basel). 2024 Oct 4;14(19):2217. doi: 10.3390/diagnostics14192217. Diagnostics (Basel). 2024. PMID: 39410621 Free PMC article.
References
-
- Payne TR, Oshima H, Okada M, Momoi N, Tobita K, Keller BB, Peng H, Huard J. A relationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts. J Am Coll Cardiol. 2007;50(17):1677–1684. doi: 10.1016/j.jacc.2007.04.100. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

