Modulation of hepatic stellate cells by Mutaflor® probiotic in non-alcoholic fatty liver disease management
- PMID: 35907883
- PMCID: PMC9338485
- DOI: 10.1186/s12967-022-03543-z
Modulation of hepatic stellate cells by Mutaflor® probiotic in non-alcoholic fatty liver disease management
Abstract
Background: NAFLD and NASH are emerging as primary causes of chronic liver disease, indicating a need for an effective treatment. Mutaflor® probiotic, a microbial treatment of interest, was effective in sustaining remission in ulcerative colitis patients.
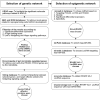
Objective: To construct a genetic-epigenetic network linked to HSC signaling as a modulator of NAFLD/NASH pathogenesis, then assess the effects of Mutaflor® on this network.
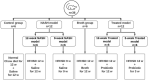
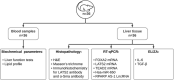
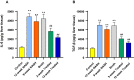
Methods: First, in silico analysis was used to construct a genetic-epigenetic network linked to HSC signaling. Second, an investigation using rats, including HFHSD induced NASH and Mutaflor® treated animals, was designed. Experimental procedures included biochemical and histopathologic analysis of rat blood and liver samples. At the molecular level, the expression of genetic (FOXA2, TEAD2, and LATS2 mRNAs) and epigenetic (miR-650, RPARP AS-1 LncRNA) network was measured by real-time PCR. PCR results were validated with immunohistochemistry (α-SMA and LATS2). Target effector proteins, IL-6 and TGF-β, were estimated by ELISA.
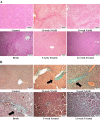
Results: Mutaflor® administration minimized biochemical and histopathologic alterations caused by NAFLD/NASH. HSC activation and expression of profibrogenic IL-6 and TGF-β effector proteins were reduced via inhibition of hedgehog and hippo pathways. Pathways may have been inhibited through upregulation of RPARP AS-1 LncRNA which in turn downregulated the expression of miR-650, FOXA2 mRNA and TEAD2 mRNA and upregulated LATS2 mRNA expression.
Conclusion: Mutaflor® may slow the progression of NAFLD/NASH by modulating a genetic-epigenetic network linked to HSC signaling. The probiotic may be a useful modality for the prevention and treatment of NAFLD/NASH.
Keywords: E. coli; Hedgehog; Hepatic Stellate cells; Hippo; Liver fibrosis; NAFLD; NASH; Probiotic.
© 2022. The Author(s).
Conflict of interest statement
The authors report no declarations of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

