Stakeholders' views on drug development: the congenital disorders of glycosylation community perspective
- PMID: 35907899
- PMCID: PMC9338569
- DOI: 10.1186/s13023-022-02460-0
Stakeholders' views on drug development: the congenital disorders of glycosylation community perspective
Abstract
Background: Congenital disorders of glycosylation (CDG) are a large family of rare genetic diseases for which therapies are virtually nonexistent. However, CDG therapeutic research has been expanding, thanks to the continuous efforts of the CDG medical/scientific and patient communities. Hence, CDG drug development is a popular research topic. The main aim of this study was to understand current and steer future CDG drug development and approval by collecting and analysing the views and experiences of the CDG community, encompassing professionals and families. An electronic (e-)survey was developed and distributed to achieve this goal.
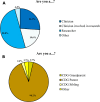
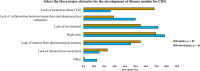
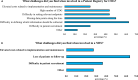
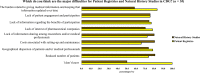
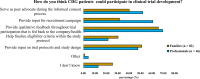
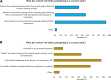
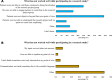
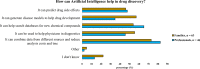
Results: A total of 128 respondents (46 CDG professionals and 82 family members), mainly from Europe and the USA, participated in this study. Most professionals (95.0%) were relatively familiar with drug development and approval processes, while CDG families revealed low familiarity levels, with 8.5% admitting to never having heard about drug development. However, both stakeholder groups agreed that patients and families make significant contributions to drug development and approval. Regarding their perceptions of and experiences with specific drug development and approval tools, namely biobanks, disease models, patient registries, natural history studies (NHS) and clinical trials (CT), the CDG community stakeholders described low use and participation, as well as variable familiarity. Additionally, CDG professionals and families shared conflicting views about CT patient engagement and related information sharing. Families reported lower levels of involvement in CT design (25.0% declared ever being involved) and information (60.0% stated having been informed) compared to professionals (60.0% and 85.7%, respectively). These contrasting perceptions were further extended to their insights and experiences with patient-centric research. Finally, the CDG community (67.4% of professionals and 54.0% of families) reported a positive vision of artificial intelligence (AI) as a drug development tool. Nevertheless, despite the high AI awareness among CDG families (76.8%), professionals described limited AI use in their research (23.9%).
Conclusions: This community-centric study sheds new light on CDG drug development and approval. It identifies educational, communication and research gaps and opportunities for CDG professionals and families that could improve and accelerate CDG therapy development.
Keywords: Congenital disorder(s) of glycosylation (CDG); Drug development; Electronic (e-)survey; Patient-reported outcome measures; People-centricity.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

