Curcumin piperidone derivatives induce anti-proliferative and anti-migratory effects in LN-18 human glioblastoma cells
- PMID: 35907913
- PMCID: PMC9338982
- DOI: 10.1038/s41598-022-16274-4
Curcumin piperidone derivatives induce anti-proliferative and anti-migratory effects in LN-18 human glioblastoma cells
Abstract
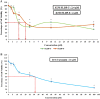
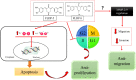
Curcumin has demonstrated potential cytotoxicity across various cell lines despite its poor bioavailability and rapid metabolism. Therefore, our group have synthesized curcuminoid analogues with piperidone derivatives, FLDP-5 and FLDP-8 to overcome these limitations. In this study, the analogues were assessed on LN-18 human glioblastoma cells in comparison to curcumin. Results from cytotoxicity assessment showed that FLDP-5 and FLDP-8 curcuminoid analogues caused death in LN-18 cells in a concentration-dependent manner after 24-h treatment with much lower IC50 values of 2.5 µM and 4 µM respectively, which were more potent compared to curcumin with IC50 of 31 µM. Moreover, a significant increase (p < 0.05) in the level of superoxide anion and hydrogen peroxide upon 2-h and 6-h treatment confirmed the oxidative stress involvement in the cell death process induced by these analogues. These analogues also showed potent anti-migratory effects through inhibition of LN-18 cells' migration and invasion. In addition, cell cycle analysis showed that these analogues are capable of inducing significant (p < 0.05) S-phase cell cycle arrest during the 24-h treatment as compared to untreated, which explained the reduced proliferation indicated by MTT assay. In conclusion, these curcuminoid analogues exhibit potent anti-cancer effects with anti-proliferative and anti-migratory properties towards LN-18 cells as compared to curcumin.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- Banu Z. Glioblastoma multiforme: a review of its pathogenesis and treatment. Int. Res. J. Pharm. 2019;9:7–12. doi: 10.7897/2230-8407.0912283. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

