Leveraging antigenic seniority for maternal vaccination to prevent mother-to-child transmission of HIV-1
- PMID: 35907918
- PMCID: PMC9338948
- DOI: 10.1038/s41541-022-00505-w
Leveraging antigenic seniority for maternal vaccination to prevent mother-to-child transmission of HIV-1
Abstract
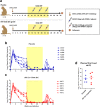
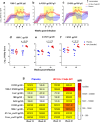
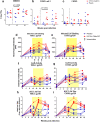
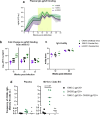
The development of a maternal HIV vaccine to synergize with current antiretroviral drug prophylaxis can overcome implementation challenges and further reduce mother-to-child transmission (MTCT) of HIV. Both the epitope-specificity and autologous neutralization capacity of maternal HIV envelope (Env)-specific antibodies have been implicated in decreased risk of MTCT of HIV. Our goal was to determine if heterologous HIV Env immunization of SHIV.C.CH505-infected, ART-suppressed female rhesus macaques (RMs) could boost autologous Env-specific antibodies. SHIV.C.CH505-infected female RMs (n = 12), began a daily ART regimen at 12 weeks post-infection (wpi), which was continued for 12 weeks. Starting 2 weeks after ART initiation, RMs received 3 monthly immunizations with HIV b.63521/1086.C gp120 or placebo (n = 6/group) vaccine with adjuvant STR8S-C. Compared to the placebo-immunized animals, Env-vaccinated, SHIV-infected RMs exhibited enhanced IgG binding, avidity, and ADCC responses against the vaccine immunogens and the autologous SHIV.C.CH505 Env. Notably, the Env-specific memory B cells elicited by heterologous vaccination were dominated by cells that recognized the SHIV.C.CH505 Env, the antigen of primary exposure. Thus, vaccination of SHIV-infected, ART-suppressed RMs with heterologous HIV Envs can augment multiple components of the antibody response against the Env antigen of primary exposure, suggesting antigenic seniority. Our results suggest that a universal maternal HIV vaccination regimen can be developed to leverage antigenic seniority in targeting the maternal autologous virus pool.
© 2022. The Author(s).
Conflict of interest statement
S.R.P. is a consultant for Merck, Moderna, Pfizer, and Dynavax vaccine programs, and has a sponsored program with Merck and Moderna. All other authors declare no competing interests.
Figures

References
-
- UNAIDS. Global HIV & AIDS Statistics — 2019 Fact Sheet 2019. (UNAIDS, 2019).
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

