Pharmacovigilance study of the association between dipeptidyl peptidase-4 inhibitors and angioedema using the FDA Adverse Event Reporting System (FAERS)
- PMID: 35907939
- PMCID: PMC9338932
- DOI: 10.1038/s41598-022-17366-x
Pharmacovigilance study of the association between dipeptidyl peptidase-4 inhibitors and angioedema using the FDA Adverse Event Reporting System (FAERS)
Abstract
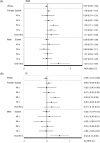
Dipeptidyl peptidase-4 (DPP-4) plays a minor role in degrading vasoactive peptides that cause angioedema when angiotensin-converting enzyme (ACE) is present and fully functional. This study investigated the association between DPP-4 inhibitors (DPP-4Is) and angioedema, including cases where the concomitant use of ACE inhibitors (ACEIs) was absent. We obtained data from the US Food and Drug Administration Adverse Event Reporting System and performed a disproportionality analysis, using the reporting odds ratio (ROR) and information component (IC) for signal detection in patients aged ≥ 40 years, stratified by age group and sex. No signal was detected for DPP-4Is when the entire dataset was analyzed. However, a signal was detected for the entire female subset group, the three stratified female groups aged ≥ 60 years, and males in their 40 s. After excluding the data of concomitant ACEI users, most ROR and IC values were lower and significant only for females in their 60 s and males aged ≥ 80 years. Regarding individual DPP-4Is signals, those detected for saxagliptin and sitagliptin in some age groups disappeared after excluding the data of ACEI users. Notably, linagliptin was the only DPP-4I where signals were detected in most female groups, regardless of age and without concomitant ACEI use. Our findings suggest that some DPP-4Is were associated with a higher reporting of angioedema as per age and sex, even in the absence of concomitant ACEI use.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

