A maternal high-fat diet induces fetal origins of NASH-HCC in mice
- PMID: 35907977
- PMCID: PMC9338981
- DOI: 10.1038/s41598-022-17501-8
A maternal high-fat diet induces fetal origins of NASH-HCC in mice
Abstract
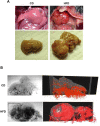
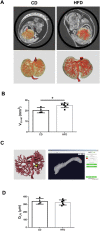
Maternal overnutrition affects offspring susceptibility to nonalcoholic steatohepatitis (NASH). Male offspring from high-fat diet (HFD)-fed dams developed a severe form of NASH, leading to highly vascular tumor formation. The cancer/testis antigen HORMA domain containing protein 1 (HORMAD1), one of 146 upregulated differentially expressed genes in fetal livers from HFD-fed dams, was overexpressed with hypoxia-inducible factor 1 alpha (HIF-1alpha) in hepatoblasts and in NASH-based hepatocellular carcinoma (HCC) in offspring from HFD-fed dams at 15 weeks old. Hypoxia substantially increased Hormad1 expression in primary mouse hepatocytes. Despite the presence of three putative hypoxia response elements within the mouse Hormad1 gene, the Hif-1alpha siRNA only slightly decreased hypoxia-induced Hormad1 mRNA expression. In contrast, N-acetylcysteine, but not rotenone, inhibited hypoxia-induced Hormad1 expression, indicating its dependency on nonmitochondrial reactive oxygen species production. Synchrotron-based phase-contrast micro-CT of the fetuses from HFD-fed dams showed significant enlargement of the liver accompanied by a consistent size of the umbilical vein, which may cause hypoxia in the fetal liver. Based on these findings, a maternal HFD induces fetal origins of NASH/HCC via hypoxia, and HORMAD1 is a potential therapeutic target for NASH/HCC.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures











References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

