The RNA-binding protein PCBP1 represses lung adenocarcinoma progression by stabilizing DKK1 mRNA and subsequently downregulating β-catenin
- PMID: 35907982
- PMCID: PMC9338556
- DOI: 10.1186/s12967-022-03552-y
The RNA-binding protein PCBP1 represses lung adenocarcinoma progression by stabilizing DKK1 mRNA and subsequently downregulating β-catenin
Abstract
Background: PolyC-RNA-binding protein 1 (PCBP1) functions as a tumour suppressor and RNA regulator that is downregulated in human cancers. Here, we aimed to reveal the biological function of PCBP1 in lung adenocarcinoma (LUAD).
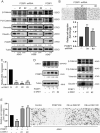
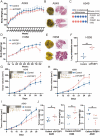
Methods: First, PCBP1 was identified as an important biomarker that maintains LUAD through The Cancer Genome Atlas (TCGA) project screening and confirmed by immunohistochemistry and qPCR. Via colony formation, CCK8, IncuCyte cell proliferation, wound healing and Transwell assays, we confirmed that PCBP1 was closely related to the proliferation and migration of LUAD cells. The downstream gene DKK1 was discovered by RNA sequencing of PCBP1 knockdown cells. The underlying mechanisms were further investigated using western blot, qPCR, RIP, RNA pulldown and mRNA stability assays.
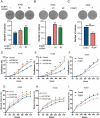
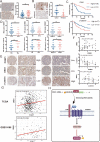
Results: We demonstrate that PCBP1 is downregulated in LUAD tumour tissues. The reduction in PCBP1 promotes the proliferation, migration and invasion of LUAD in vitro and in vivo. Mechanistically, the RNA-binding protein PCBP1 represses LUAD by stabilizing DKK1 mRNA. Subsequently, decreased expression of the DKK1 protein relieves the inhibitory effect on the Wnt/β-catenin signalling pathway. Taken together, these results show that PCBP1 acts as a tumour suppressor gene, inhibiting the tumorigenesis of LUAD.
Conclusions: We found that PCBP1 inhibits LUAD development by upregulating DKK1 to inactivate the Wnt/β-catenin pathway. Our findings highlight the potential of PCBP1 as a promising therapeutic target.
Keywords: DKK1; LUAD; PCBP1; Wnt signalling pathway.
© 2022. The Author(s).
Conflict of interest statement
Authors declare no conflicts of interest for this article.
Figures



Similar articles
-
STAT1-induced upregulation of LINC00467 promotes the proliferation migration of lung adenocarcinoma cells by epigenetically silencing DKK1 to activate Wnt/β-catenin signaling pathway.Biochem Biophys Res Commun. 2019 Jun 18;514(1):118-126. doi: 10.1016/j.bbrc.2019.04.107. Epub 2019 Apr 23. Biochem Biophys Res Commun. 2019. PMID: 31027730
-
CircRNA has_circ_0006427 suppresses the progression of lung adenocarcinoma by regulating miR-6783-3p/DKK1 axis and inactivating Wnt/β-catenin signaling pathway.Biochem Biophys Res Commun. 2019 Jan 1;508(1):37-45. doi: 10.1016/j.bbrc.2018.11.079. Epub 2018 Nov 20. Biochem Biophys Res Commun. 2019. PMID: 30470570
-
Junctional adhesion molecule-like protein promotes tumor progression via the Wnt/β-catenin signaling pathway in lung adenocarcinoma.J Transl Med. 2022 Jun 7;20(1):260. doi: 10.1186/s12967-022-03457-w. J Transl Med. 2022. PMID: 35672776 Free PMC article.
-
LncRNA PCBP1-AS1 correlated with the functional states of cancer cells and inhibited lung adenocarcinoma metastasis by suppressing the EMT progression.Carcinogenesis. 2021 Jul 16;42(7):931-939. doi: 10.1093/carcin/bgab047. Carcinogenesis. 2021. PMID: 34107009
-
Decoding poly (RC)-binding protein 1 (PCBP1), the underrated guard at the foothill of ferroptosis.Pathol Res Pract. 2025 Feb;266:155771. doi: 10.1016/j.prp.2024.155771. Epub 2024 Dec 12. Pathol Res Pract. 2025. PMID: 39700662 Review.
Cited by
-
RNA binding proteins (RBPs) on genetic stability and diseases.Glob Med Genet. 2024 Nov 30;12(1):100032. doi: 10.1016/j.gmg.2024.100032. eCollection 2025 Mar. Glob Med Genet. 2024. PMID: 39925443 Free PMC article. Review.
-
The role of RNA binding proteins in cancer biology: A focus on FMRP.Genes Dis. 2024 Dec 21;12(4):101493. doi: 10.1016/j.gendis.2024.101493. eCollection 2025 Jul. Genes Dis. 2024. PMID: 40271197 Free PMC article. Review.
-
Investigating the Function of Human Jumping Translocation Breakpoint Protein (hJTB) and Its Interacting Partners through In-Solution Proteomics of MCF7 Cells.Molecules. 2022 Nov 28;27(23):8301. doi: 10.3390/molecules27238301. Molecules. 2022. PMID: 36500393 Free PMC article.
-
LncRNA MIR210HG promotes the proliferation of colon cancer cells by inhibiting ferroptosis through binding to PCBP1.Sci Rep. 2025 Jan 6;15(1):871. doi: 10.1038/s41598-025-85321-7. Sci Rep. 2025. PMID: 39757305 Free PMC article.
-
A nicotinamide metabolism-related gene signature for predicting immunotherapy response and prognosis in lung adenocarcinoma patients.PeerJ. 2025 Feb 27;13:e18991. doi: 10.7717/peerj.18991. eCollection 2025. PeerJ. 2025. PMID: 40034678 Free PMC article.
References
-
- Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

