(+)-Usnic acid and its salts, inhibitors of SARS-CoV-2, identified by using in silico methods and in vitro assay
- PMID: 35908082
- PMCID: PMC9338942
- DOI: 10.1038/s41598-022-17506-3
(+)-Usnic acid and its salts, inhibitors of SARS-CoV-2, identified by using in silico methods and in vitro assay
Abstract
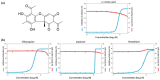
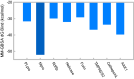
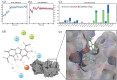
The pandemic caused by severe acute respiratory Coronavirus-2 (SARS-CoV-2) has been ongoing for over two years, and treatment for COVID-19, other than monoclonal antibodies, is urgently required. Accordingly, we have investigated the inhibitors of SARS-CoV-2 protein targets by high-throughput virtual screening using a marine natural products database. Considering the calculated molecular properties and availability of the compounds, (+)-usnic acid was selected as a suitable hit. In the in vitro antiviral assay of (+)-usnic acid by the immunofluorescence method, IC50 was 7.99 μM, which is similar to that of remdesivir used as a positive control. The generalized Born and surface area continuum solvation (MM/GBSA) method was performed to find the potent target of (+)-usnic acid, and the Mpro protein showed the most prominent value, -52.05 kcal/mol, among other SARS-CoV-2 protein targets. Thereafter, RMSD and protein-ligand interactions were profiled using molecular dynamics (MD) simulations. Sodium usnate (NaU) improved in vitro assay results with an IC50 of 5.33 μM and a selectivity index (SI) of 9.38. Additionally, when (+)-usnic acid was assayed against SARS-CoV-2 variants, it showed enhanced efficacy toward beta variants with an IC50 of 2.92 μM and SI of 11.1. We report the in vitro anti-SARS-CoV-2 efficacy of (+)-usnic acid in this study and propose that it has the potential to be developed as a COVID-19 treatment if its in vivo efficacy has been confirmed.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Targeting SARS-CoV-2 main protease: structure based virtual screening, in silico ADMET studies and molecular dynamics simulation for identification of potential inhibitors.J Biomol Struct Dyn. 2022 May;40(8):3609-3625. doi: 10.1080/07391102.2020.1848636. Epub 2020 Nov 23. J Biomol Struct Dyn. 2022. PMID: 33226303 Free PMC article.
-
Integrated bioinformatics-cheminformatics approach toward locating pseudo-potential antiviral marine alkaloids against SARS-CoV-2-Mpro.Proteins. 2022 Sep;90(9):1617-1633. doi: 10.1002/prot.26341. Epub 2022 Apr 13. Proteins. 2022. PMID: 35384056 Free PMC article.
-
Development of Effective Therapeutic Molecule from Natural Sources against Coronavirus Protease.Int J Mol Sci. 2021 Aug 30;22(17):9431. doi: 10.3390/ijms22179431. Int J Mol Sci. 2021. PMID: 34502340 Free PMC article.
-
In silico drug discovery of major metabolites from spices as SARS-CoV-2 main protease inhibitors.Comput Biol Med. 2020 Nov;126:104046. doi: 10.1016/j.compbiomed.2020.104046. Epub 2020 Oct 8. Comput Biol Med. 2020. PMID: 33065388 Free PMC article.
-
Identification of potential plant-based inhibitor against viral proteases of SARS-CoV-2 through molecular docking, MM-PBSA binding energy calculations and molecular dynamics simulation.Mol Divers. 2021 Aug;25(3):1963-1977. doi: 10.1007/s11030-021-10211-9. Epub 2021 Apr 15. Mol Divers. 2021. PMID: 33856591 Free PMC article.
Cited by
-
Effects of Usnic Acid to Prevent Infections by Creating a Protective Barrier in an In Vitro Study.Int J Mol Sci. 2023 Feb 12;24(4):3695. doi: 10.3390/ijms24043695. Int J Mol Sci. 2023. PMID: 36835105 Free PMC article.
-
Advances in Research on Bioactivity, Toxicity, Metabolism, and Pharmacokinetics of Usnic Acid In Vitro and In Vivo.Molecules. 2022 Nov 2;27(21):7469. doi: 10.3390/molecules27217469. Molecules. 2022. PMID: 36364296 Free PMC article. Review.
-
(+)-Usnic Acid and Its Derivatives as Inhibitors of a Wide Spectrum of SARS-CoV-2 Viruses.Viruses. 2022 Sep 29;14(10):2154. doi: 10.3390/v14102154. Viruses. 2022. PMID: 36298709 Free PMC article.
-
Safety in Rats of a Novel Nasal Spray Formulation for the Prevention of Airborne Viral Infections.Pharmaceutics. 2023 Feb 9;15(2):591. doi: 10.3390/pharmaceutics15020591. Pharmaceutics. 2023. PMID: 36839913 Free PMC article.
-
Usnic Acid-Mediated Exchange of Protons for Divalent Metal Cations across Lipid Membranes: Relevance to Mitochondrial Uncoupling.Int J Mol Sci. 2022 Dec 19;23(24):16203. doi: 10.3390/ijms232416203. Int J Mol Sci. 2022. PMID: 36555847 Free PMC article.
References
-
- WHO. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ (2021).
-
- Ruan, L. & Zeng, G. in Emerging Infections in Asia, 75–96 (Springer, 2008).
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

