Disease progression, treatments, hospitalization, and clinical outcomes in acute GVHD: a multicenter chart review
- PMID: 35908108
- PMCID: PMC9576589
- DOI: 10.1038/s41409-022-01764-w
Disease progression, treatments, hospitalization, and clinical outcomes in acute GVHD: a multicenter chart review
Erratum in
-
Correction to: Disease progression, treatments, hospitalization, and clinical outcomes in acute GVHD: a multicenter chart review.Bone Marrow Transplant. 2023 Feb;58(2):237. doi: 10.1038/s41409-022-01857-6. Bone Marrow Transplant. 2023. PMID: 36369478 Free PMC article. No abstract available.
Abstract
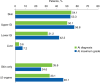
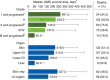
Acute graft-versus-host disease (GVHD) remains a barrier to successful allogeneic hematopoietic cell transplantation (HCT) outcomes. This multicenter, retrospective chart review describes disease progression, treatment patterns, hospitalizations, and clinical outcomes among 475 patients (≥12 years old) who developed grades II-IV acute GVHD after their first HCT (January 2014-June 2016). Median (interquartile range) age at HCT was 55 (44-63) years. From the date of acute GVHD diagnosis, 190 patients (40.0%) experienced progression to more severe disease and/or developed new organ involvement. Among 431 patients with grades II-IV acute GVHD at diagnosis, 73.1% received first-line systemic corticosteroids. During follow-up (median 524 days since acute GVHD diagnosis), 23.4% of patients had an increase in steroid dose and 44.4% were unable to taper below 10 mg/day. Over half of patients (54.9%) required ≥1 hospital readmission within 100 days post-HCT (≥2 readmissions in 22.3%); mean inpatient length of stay upon readmission was 27.5 days. Approximately half of patients (52.8%) died during follow-up; 1-year overall mortality from date of acute GVHD diagnosis and nonrelapse mortality rates were 35.2% and 25.5%, respectively. Overall, patients who developed acute GVHD following HCT had poor clinical outcomes, highlighting the unmet need for early and effective treatment strategies.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
SGH has served as a consultant for Bristol Myers Squibb, CSL Behring, Generon Corporation, and Incyte Corporation. JY, DP, and AN are employees and shareholders of Incyte Corporation. HKC has nothing to disclose. JT is an employee of Asclepius Analytics, which received funding for this project from Incyte Corporation. JG previously received consulting fees from and is currently an employee and shareholder of Incyte Corporation. HJD has received consulting fees from Incyte Corporation.
Figures

References
-
- D’Souza A, Fretham C, Center for International Blood & Marrow Transplant Research. Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR summary slides, 2019. Available at: https://www.cibmtr.org. Accessed April 14, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

