Long-term efficacy and safety of inotersen for hereditary transthyretin amyloidosis: NEURO-TTR open-label extension 3-year update
- PMID: 35908242
- PMCID: PMC9618524
- DOI: 10.1007/s00415-022-11276-8
Long-term efficacy and safety of inotersen for hereditary transthyretin amyloidosis: NEURO-TTR open-label extension 3-year update
Abstract
Background: Hereditary transthyretin amyloidosis (hATTR/ATTRv) results from the deposition of misfolded transthyretin (TTR) throughout the body, including peripheral nerves. Inotersen, an antisense oligonucleotide inhibitor of hepatic TTR production, demonstrated a favorable efficacy and safety profile in patients with the polyneuropathy associated with hATTR in the NEURO-TTR (NCT01737398) study. We report longer-term efficacy and safety data for inotersen, with a median treatment exposure of 3 years.
Methods: Patients who satisfactorily completed NEURO-TTR were enrolled in its open-label extension (OLE) study. Efficacy assessments included the modified Neuropathy Impairment Score + 7 (mNIS + 7), Norfolk Quality of Life-Diabetic Neuropathy (Norfolk QoL-DN) questionnaire total score, and the Short Form 36 (SF-36v2) Health Survey Physical Component Summary score. Safety and tolerability were also assessed. Efficacy is reported for patients living in Europe and North America (this cohort completed the study approximately 9 months before the remaining group of patients outside these regions); safety is reported for the full safety dataset, comprising patients living in Europe, North America, and Latin America/Australasia. This study is registered with ClinicalTrials.gov, identifier NCT02175004.
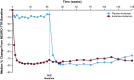
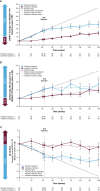
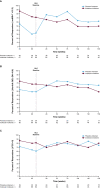
Results: In the Europe and North America cohort of the NEURO-TTR study, 113/141 patients (80.1%) completed the study, and 109 patients participated in the OLE study. A total of 70 patients continued to receive inotersen (inotersen-inotersen) and 39 switched from placebo to inotersen (placebo-inotersen). The placebo-inotersen group demonstrated sustained improvement in neurological disease progression as measured by mNIS + 7, compared with predicted worsening based on projection of the NEURO-TTR placebo data (estimated natural history). The inotersen-inotersen group demonstrated sustained benefit, as measured by mNIS + 7, Norfolk QoL-DN, and SF-36v2, compared with estimated natural history as well as compared with the placebo-inotersen group. With a maximum exposure of 6.2 years, inotersen was not associated with any additional safety concerns or increased toxicity in the OLE study. Platelet and renal monitoring were effective in reducing the risk of severe adverse events in the OLE study.
Conclusion: Inotersen treatment for > 3 years slowed progression of the polyneuropathy associated with hATTR, and no new safety signals were observed.
Keywords: Clinical trial; Familial amyloid polyneuropathy; Hereditary transthyretin amyloidosis; Inotersen; Peripheral neuropathies; Polyneuropathy.
© 2022. The Author(s).
Conflict of interest statement
TB reports being an advisory board member for Akcea Therapeutics, Pfizer, and Alnylam Pharmaceuticals; study investigator for Ionis Pharmaceuticals, Inc., and Alnylam Pharmaceuticals; and speaker for Alnylam Pharmaceuticals; and receiving honoraria for speaking for Akcea Therapeutics. TC reports receiving financial support to attend scientific meetings from Pfizer, Alnylam, Ionis, Akcea, and Biogen. AKW reports being study investigator, consultant, and speaker for Ionis Pharmaceuticals, Inc.; consultant for Pfizer; and study investigator for Alnylam. MJP reports receiving honoraria from Pfizer and Alnylam Pharmaceuticals, Inc. PJD reports receiving training and consulting honoraria from Ionis Pharmaceuticals, Inc., and Alnylam Pharmaceuticals. JLB reports receiving honoraria from Ionis Pharmaceuticals, Inc., and Alnylam Pharmaceuticals; and being a study investigator for Ionis Pharmaceuticals, Inc., Alnylam Pharmaceuticals, Eidos BridgeBio, and Pfizer. BD reports being a consultant/advisory board member for Eidos and Alnylam Pharmaceuticals. PG declares no competing interests. CW reports being an advisory board member for Alnylam and Akcea Therapeutics. IC reports being a consultant for Alnylam and Pfizer; receiving honoraria for Alnylam, Pfizer, and Sanofi; and being on speaker bureaus for Alnylam, Pfizer, and Sanofi. VP-B reports receiving consulting honoraria for Alnylam Pharmaceuticals, Ionis, and Pfizer and travel honoraria from Ionis. GM declares no competing interests. LO reports receiving speaker honoraria from Akcea Therapeutics, Alnylam Pharmaceuticals, SOBI, and Pfizer. JMCP declares no competing interests. JG declares no competing interests. AVK reports receiving honoraria and fees for lectures and speakers bureaus from Alnylam Pharmaceuticals, Akcea Therapeutics, and Pfizer Inc. AM reports receiving speaker fees and consulting honoraria from Alnylam Pharmaceuticals, Akcea Therapeutics, and Pfizer Inc. LG reports receiving speaker fees and consulting honoraria from Pfizer. AN is a former employee of Akcea Therapeutics, a subsidiary of Ionis Pharmaceuticals, Inc. KO is a former employee of Ionis Pharmaceuticals, Inc. PA is a former employee of Ionis Pharmaceuticals, Inc./Akcea Therapeutics. MDB reports being study investigator for Ionis Pharmaceuticals, Inc. MG reports receiving consulting honoraria from Ionis, AbbVie, Alnylam, Amgen, Annexon, Appellis, Celgene, Janssen, Medscape, Physicians Education Resource, Prothena, Research to Practice, Teva, and Spectrum, and grants from Spectrum.
Figures
Similar articles
-
Early data on long-term efficacy and safety of inotersen in patients with hereditary transthyretin amyloidosis: a 2-year update from the open-label extension of the NEURO-TTR trial.Eur J Neurol. 2020 Aug;27(8):1374-1381. doi: 10.1111/ene.14285. Epub 2020 May 29. Eur J Neurol. 2020. PMID: 32343462 Free PMC article. Clinical Trial.
-
Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis.N Engl J Med. 2018 Jul 5;379(1):22-31. doi: 10.1056/NEJMoa1716793. N Engl J Med. 2018. PMID: 29972757 Clinical Trial.
-
Early Data on Long-term Impact of Inotersen on Quality-of-Life in Patients with Hereditary Transthyretin Amyloidosis Polyneuropathy: Open-Label Extension of NEURO-TTR.Neurol Ther. 2021 Dec;10(2):865-886. doi: 10.1007/s40120-021-00268-x. Epub 2021 Aug 5. Neurol Ther. 2021. PMID: 34355354 Free PMC article.
-
Pharmacological treatment for familial amyloid polyneuropathy.Cochrane Database Syst Rev. 2020 Apr 20;4(4):CD012395. doi: 10.1002/14651858.CD012395.pub2. Cochrane Database Syst Rev. 2020. PMID: 32311072 Free PMC article.
-
Inotersen for the treatment of adults with polyneuropathy caused by hereditary transthyretin-mediated amyloidosis.Expert Rev Clin Pharmacol. 2019 Aug;12(8):701-711. doi: 10.1080/17512433.2019.1635008. Epub 2019 Jul 3. Expert Rev Clin Pharmacol. 2019. PMID: 31268366 Review.
Cited by
-
Hereditary Transthyretin Amyloidosis (hATTR) with Polyneuropathy Clusters Are Located in Ancient Mining Districts: A Possible Geochemical Origin of the Disease.Biomolecules. 2024 Jun 3;14(6):652. doi: 10.3390/biom14060652. Biomolecules. 2024. PMID: 38927056 Free PMC article. Review.
-
Hemodynamics in Left-Sided Cardiomyopathies.Rev Cardiovasc Med. 2024 Dec 24;25(12):455. doi: 10.31083/j.rcm2512455. eCollection 2024 Dec. Rev Cardiovasc Med. 2024. PMID: 39742240 Free PMC article. Review.
-
Anti-MAG neuropathy: historical aspects, clinical-pathological correlations, and considerations for future therapeutical trials.Arq Neuropsiquiatr. 2024 Jun;82(6):1-7. doi: 10.1055/s-0043-1777728. Epub 2024 Feb 7. Arq Neuropsiquiatr. 2024. PMID: 38325389 Free PMC article. Review.
-
Unravelling the myriad physiologic roles of transthyretin: critical considerations for treating transthyretin amyloidosis.Ann Med. 2025 Dec;57(1):2536755. doi: 10.1080/07853890.2025.2536755. Epub 2025 Jul 27. Ann Med. 2025. PMID: 40717228 Free PMC article. Review.
-
World Heart Federation Consensus on Transthyretin Amyloidosis Cardiomyopathy (ATTR-CM).Glob Heart. 2023 Oct 26;18(1):59. doi: 10.5334/gh.1262. eCollection 2023. Glob Heart. 2023. PMID: 37901600 Free PMC article. Review.
References
-
- Gertz MA. Hereditary ATTR amyloidosis: burden of illness and diagnostic challenges. Am J Manag Care. 2017;23:S107–S112102. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

