The Alzheimer's Association appropriate use recommendations for blood biomarkers in Alzheimer's disease
- PMID: 35908251
- PMCID: PMC10087669
- DOI: 10.1002/alz.12756
The Alzheimer's Association appropriate use recommendations for blood biomarkers in Alzheimer's disease
Abstract
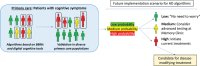
Blood-based markers (BBMs) have recently shown promise to revolutionize the diagnostic and prognostic work-up of Alzheimer's disease (AD), as well as to improve the design of interventional trials. Here we discuss in detail further research needed to be performed before widespread use of BBMs. We already now recommend use of BBMs as (pre-)screeners to identify individuals likely to have AD pathological changes for inclusion in trials evaluating disease-modifying therapies, provided the AD status is confirmed with positron emission tomography (PET) or cerebrospinal fluid (CSF) testing. We also encourage studying longitudinal BBM changes in ongoing as well as future interventional trials. However, BBMs should not yet be used as primary endpoints in pivotal trials. Further, we recommend to cautiously start using BBMs in specialized memory clinics as part of the diagnostic work-up of patients with cognitive symptoms and the results should be confirmed whenever possible with CSF or PET. Additional data are needed before use of BBMs as stand-alone diagnostic AD markers, or before considering use in primary care.
Keywords: Alzheimer's disease; appropriate use recommendations; blood-based biomarkers; diagnosis; prognosis.
© 2022 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Author disclosures are available in the supporting information.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG054029/AG/NIA NIH HHS/United States
- U19 AG063911/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- R35 AG072362/AG/NIA NIH HHS/United States
- R01 AG053798/AG/NIA NIH HHS/United States
- U01 AG045390/AG/NIA NIH HHS/United States
- U54 NS092089/NS/NINDS NIH HHS/United States
- R01 AG061848/AG/NIA NIH HHS/United States
- R01 AG063689/AG/NIA NIH HHS/United States
- R56 AG057195/AG/NIA NIH HHS/United States
- U01 AG057195/AG/NIA NIH HHS/United States
- U24 AG057437/AG/NIA NIH HHS/United States
- P01 AG036694/AG/NIA NIH HHS/United States
- R01 AG038791/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

