Neural substrates of motivational dysfunction across neuropsychiatric conditions: Evidence from meta-analysis and lesion network mapping
- PMID: 35908312
- PMCID: PMC9720091
- DOI: 10.1016/j.cpr.2022.102189
Neural substrates of motivational dysfunction across neuropsychiatric conditions: Evidence from meta-analysis and lesion network mapping
Abstract
Motivational dysfunction constitutes one of the fundamental dimensions of psychopathology cutting across traditional diagnostic boundaries. However, it is unclear whether there is a common neural circuit responsible for motivational dysfunction across neuropsychiatric conditions. To address this issue, the current study combined a meta-analysis on psychiatric neuroimaging studies of reward/loss anticipation and consumption (4308 foci, 438 contrasts, 129 publications) with a lesion network mapping approach (105 lesion cases). Our meta-analysis identified transdiagnostic hypoactivation in the ventral striatum (VS) for clinical/at-risk conditions compared to controls during the anticipation of both reward and loss. Moreover, the VS subserves a key node in a distributed brain network which encompasses heterogeneous lesion locations causing motivation-related symptoms. These findings do not only provide the first meta-analytic evidence of shared neural alternations linked to anticipatory motivation-related deficits, but also shed novel light on the role of VS dysfunction in motivational impairments in terms of both network integration and psychological functions. Particularly, the current findings suggest that motivational dysfunction across neuropsychiatric conditions is rooted in disruptions of a common brain network anchored in the VS, which contributes to motivational salience processing rather than encoding positive incentive values.
Keywords: Lesion network mapping; Meta-analysis; Monetary incentive delay task; Motivation; Ventral striatum.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors are unaware of any conflicts of interest, financial or otherwise.
Figures
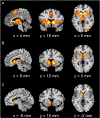
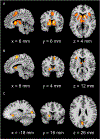
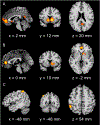
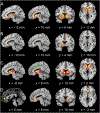
References
-
- Arrondo G, Segarra N, Metastasio A, Ziauddeen H, Spencer J, Reinders NR, … Murray GK (2015). Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: A replicated cross-diagnostic finding. Frontiers in Psychology, 6, 1280. 10.3389/fpsyg.2015.01280 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

