Ca2+ signalling system initiated by endoplasmic reticulum stress stimulates PERK activation
- PMID: 35908318
- PMCID: PMC9982837
- DOI: 10.1016/j.ceca.2022.102622
Ca2+ signalling system initiated by endoplasmic reticulum stress stimulates PERK activation
Abstract
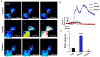
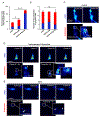
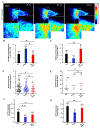
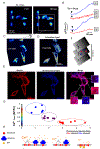
The accumulation of unfolded proteins within the Endoplasmic Reticulum (ER) activates a signal transduction pathway termed the unfolded protein response (UPR), which attempts to restore ER homoeostasis. If this cannot be done, UPR signalling ultimately induces apoptosis. Ca2+ depletion in the ER is a potent inducer of ER stress. Despite the ubiquity of Ca2+ as an intracellular messenger, the precise mechanism(s) by which Ca2+ release affects the UPR remains unknown. Tethering a genetically encoded Ca2+ indicator (GCamP6) to the ER membrane revealed novel Ca2+ signalling events initiated by Ca2+ microdomains in human astrocytes under ER stress, induced by tunicamycin (Tm), an N-glycosylation inhibitor, as well as in a cell model deficient in all three inositol triphosphate receptor isoforms. Pharmacological and molecular studies indicate that these local events are mediated by translocons and that the Ca2+ microdomains impact (PKR)-like-ER kinase (PERK), an UPR sensor, activation. These findings reveal the existence of a Ca2+ signal mechanism by which stressor-mediated Ca2+ release regulates ER stress.
Keywords: (PKR)-like-ER kinase (PERK); Calcium signalling; Inositol triphosphate receptor; Translocon; Unfolded protein response.
Copyright © 2022. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest
The authors declare no competing financial interests.
Figures





Similar articles
-
Protein-rich foods, sea foods, and gut microbiota amplify immune responses in chronic diseases and cancers - Targeting PERK as a novel therapeutic strategy for chronic inflammatory diseases, neurodegenerative disorders, and cancer.Pharmacol Ther. 2024 Mar;255:108604. doi: 10.1016/j.pharmthera.2024.108604. Epub 2024 Feb 13. Pharmacol Ther. 2024. PMID: 38360205 Free PMC article. Review.
-
Melatonin Induces PERK-ATF4 Unfolded Protein Response and Apoptosis in Human Choriocarcinoma Cells.J Pineal Res. 2025 Sep;77(5):e70072. doi: 10.1111/jpi.70072. J Pineal Res. 2025. PMID: 40873119 Free PMC article.
-
USP18 attenuates endoplasmic reticulum stress via the PERK-eIF2α-ATF4 axis to reduce apoptosis in hepatocellular carcinoma cells.Sci Rep. 2025 May 5;15(1):15659. doi: 10.1038/s41598-025-00540-2. Sci Rep. 2025. PMID: 40325079 Free PMC article.
-
PERK signaling maintains hematopoietic stem cell pool integrity under endoplasmic reticulum stress by promoting proliferation.Blood. 2025 Aug 14;146(7):806-818. doi: 10.1182/blood.2024027846. Blood. 2025. PMID: 40435413
-
Glucose sensing and the unfolded protein response.FEBS J. 2025 Jul;292(14):3581-3595. doi: 10.1111/febs.70113. Epub 2025 Apr 24. FEBS J. 2025. PMID: 40272086 Review.
Cited by
-
The mutual interaction of TRPC5 channel with polycystin proteins.Korean J Physiol Pharmacol. 2025 Jan 1;29(1):93-108. doi: 10.4196/kjpp.24.265. Epub 2024 Nov 11. Korean J Physiol Pharmacol. 2025. PMID: 39523478 Free PMC article.
-
Islet Transplantation: Current Limitations and Challenges for Successful Outcomes.Cells. 2024 Oct 28;13(21):1783. doi: 10.3390/cells13211783. Cells. 2024. PMID: 39513890 Free PMC article. Review.
-
Sec61 complex/translocon: The role of an atypical ER Ca2+-leak channel in health and disease.Front Physiol. 2022 Oct 6;13:991149. doi: 10.3389/fphys.2022.991149. eCollection 2022. Front Physiol. 2022. PMID: 36277220 Free PMC article. Review.
-
Editorial: The evolving picture of Ca2+ leak from endoplasmic reticulum in health and diseases.Front Physiol. 2023 Mar 27;14:1182455. doi: 10.3389/fphys.2023.1182455. eCollection 2023. Front Physiol. 2023. PMID: 37051023 Free PMC article. No abstract available.
-
NLRP3 inflammasome: structure, mechanism, drug-induced organ toxicity, therapeutic strategies, and future perspectives.RSC Med Chem. 2025 May 13. doi: 10.1039/d5md00167f. Online ahead of print. RSC Med Chem. 2025. PMID: 40370650 Free PMC article. Review.
References
-
- Sneyd J, Keizer J, Sanderson MJ, Mechanisms of calcium oscillations and waves: a quantitative analysis, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 9(14) (1995) 1463–72. - PubMed
-
- Berridge MJ, Bootman MD, Roderick HL, Calcium signalling: dynamics, homeostasis and remodelling, Nature reviews. Molecular cell biology 4(7) (2003) 517–29. - PubMed
-
- Camacho P, Lechleiter JD, Spiral calcium waves: implications for signalling, Ciba Foundation symposium 188 (1995) 66–77; discussion 78-84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

