Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis
- PMID: 35908569
- PMCID: PMC9333998
- DOI: 10.1016/S0140-6736(22)01109-6
Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis
Erratum in
-
Department of Error.Lancet. 2022 Oct 1;400(10358):1102. doi: 10.1016/S0140-6736(22)01866-9. Lancet. 2022. PMID: 36183728 Free PMC article. No abstract available.
Abstract
Background: We aimed to evaluate the use of baricitinib, a Janus kinase (JAK) 1-2 inhibitor, for the treatment of patients admitted to hospital with COVID-19.
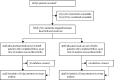
Methods: This randomised, controlled, open-label, platform trial (Randomised Evaluation of COVID-19 Therapy [RECOVERY]), is assessing multiple possible treatments in patients hospitalised with COVID-19 in the UK. Eligible and consenting patients were randomly allocated (1:1) to either usual standard of care alone (usual care group) or usual care plus baricitinib 4 mg once daily by mouth for 10 days or until discharge if sooner (baricitinib group). The primary outcome was 28-day mortality assessed in the intention-to-treat population. A meta-analysis was done, which included the results from the RECOVERY trial and all previous randomised controlled trials of baricitinib or other JAK inhibitor in patients hospitalised with COVID-19. The RECOVERY trial is registered with ISRCTN (50189673) and ClinicalTrials.gov (NCT04381936) and is ongoing.
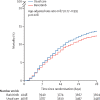
Findings: Between Feb 2 and Dec 29, 2021, from 10 852 enrolled, 8156 patients were randomly allocated to receive usual care plus baricitinib versus usual care alone. At randomisation, 95% of patients were receiving corticosteroids and 23% were receiving tocilizumab (with planned use within the next 24 h recorded for a further 9%). Overall, 514 (12%) of 4148 patients allocated to baricitinib versus 546 (14%) of 4008 patients allocated to usual care died within 28 days (age-adjusted rate ratio 0·87; 95% CI 0·77-0·99; p=0·028). This 13% proportional reduction in mortality was somewhat smaller than that seen in a meta-analysis of eight previous trials of a JAK inhibitor (involving 3732 patients and 425 deaths), in which allocation to a JAK inhibitor was associated with a 43% proportional reduction in mortality (rate ratio 0·57; 95% CI 0·45-0·72). Including the results from RECOVERY in an updated meta-analysis of all nine completed trials (involving 11 888 randomly assigned patients and 1485 deaths) allocation to baricitinib or another JAK inhibitor was associated with a 20% proportional reduction in mortality (rate ratio 0·80; 95% CI 0·72-0·89; p<0·0001). In RECOVERY, there was no significant excess in death or infection due to non-COVID-19 causes and no significant excess of thrombosis, or other safety outcomes.
Interpretation: In patients hospitalised with COVID-19, baricitinib significantly reduced the risk of death but the size of benefit was somewhat smaller than that suggested by previous trials. The total randomised evidence to date suggests that JAK inhibitors (chiefly baricitinib) reduce mortality in patients hospitalised for COVID-19 by about one-fifth.
Funding: UK Research and Innovation (Medical Research Council) and National Institute of Health Research.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests or financial relationships relevant to the submitted work. No form of payment was given to anyone to produce the manuscript. All authors have completed and submitted the International Committee of Journal Editors form for disclosure of potential conflicts of interest. The Nuffield Department of Population Health at the University of Oxford has a staff policy of not accepting honoraria or consultancy fees directly or indirectly from industry.
Figures


Comment in
-
Baricitinib in COVID-19: a coming-of-age from artificial intelligence to reducing mortality.Lancet. 2022 Jul 30;400(10349):338-339. doi: 10.1016/S0140-6736(22)01295-8. Lancet. 2022. PMID: 35908561 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- G108/613/MRC_/Medical Research Council/United Kingdom
- MC_UU_00002/14/MRC_/Medical Research Council/United Kingdom
- MC_PC_21051/MRC_/Medical Research Council/United Kingdom
- MR/R015600/1/MRC_/Medical Research Council/United Kingdom
- 211153/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- MC_UU_00017/3/MRC_/Medical Research Council/United Kingdom
- MC_U137686860/MRC_/Medical Research Council/United Kingdom
- CH/09/002/26360/BHF_/British Heart Foundation/United Kingdom
- CS/18/2/33719/BHF_/British Heart Foundation/United Kingdom
- MC_PC_20062/MRC_/Medical Research Council/United Kingdom
- MC_PC_19056/MRC_/Medical Research Council/United Kingdom
- G1002605/MRC_/Medical Research Council/United Kingdom
- SP/12/2/29422/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Research Materials

